Effect of Soymilk Intake on Diet Management and Blood Biochemistry in Diabetes Patients
 ; Hyo-Jeong Hwang1
; Hyo-Jeong Hwang1 ; Soon-Hee Park2
; Soon-Hee Park2 ; Kwang-Jin Chon3
; Kwang-Jin Chon3 ; Chung-Hwa Song4
; Chung-Hwa Song4 ; Dae-Gyun Moon4
; Dae-Gyun Moon4
Abstract
In this study, we selected soymilk, developed by Sahmyook Foods, as a snack substitute to provide balanced nutrition to elderly patients with diabetes. We confirmed the effect of soymilk consumption on diet management and blood biochemistry in these patients. The percentage of elderly male patients reporting a regular consumption of meat, fish, eggs, beans, and tofu decreased. Among elderly women with diabetes, there were significant increases in the intake of various nutrients, including vitamins (e.g., A, E, B1, B2, B6, B12, C, niacin, and folic acid) and minerals (e.g., calcium, iron, zinc, potassium, selenium, and dietary fiber) (p<0.05). Furthermore, blood glucose, glycosylated hemoglobin (HbA1c), and cholesterol levels significantly decreased in both elderly males and females following consumption of the diabetes control diet (p<0.05). Therefore, incorporating soymilk into the diet of elderly individuals with diabetes may help alleviate nutritional deficiencies and imbalances, given its functional properties that contribute to overall well-being.
Keywords:
soymilk, diabetes, nutrient intake status, blood analysisINTRODUCTION
Changes in modern eating habits and decreased physical activity may have led to the increasing incidence of diabetes (Kim MH et al 2023). Type 2 diabetes has been reported as a risk factor for further worsening of patient conditions, particularly in those with COVID-19 infection (Nassar M et al 2021). This may be due to aggressive viral replication caused increased glucose concentration, neutrophil degranulation, impaired complement activation, excessive airway secretion of pro-inflammatory cytokines, and reduced viral clearance (Chatterjee P et al 2020; Lee HI et al 2023). The International Diabetes Federation predicted that by 2021, 537 million of the global population aged 20—79 years would have diabetes, and that the number would increase to 783 million by 2045 (Sun H et al 2022; Lee HI et al 2023). In addition, according to the 2020 National Health and Nutrition Examination Survey, the prevalence of diabetes among adults aged 30 years or older in Korea was 16.7%;, in the case of the those aged 65 years or older, it was reported to be 30.1% (Kim MH et al 2023).
Diabetes is a disease in which blood sugar levels increase due to impairments in carbohydrate metabolism. Type 2 diabetes is mostly caused by tissue resistance to insulin. Diabetes is characterized by reduced insulin sensitivity, reduced glucose absorption into tissues, and increased glucose production in the liver (Guillausseau PJ et al 2008; Lee HA et al 2020). When insulin secretion is delayed or insufficient, blood glucose utilization decreases in multiple organ systems, such as the liver, and muscle. Moreover glucose production in the liver fails to cease, thus resulting in insulin resistance and abnormal glucose and lipid metabolism (Guillausseau PJ et al 2008; Kim MH et al 2023). The glucose-fatty acid cycle was proposed as an increase in intramuscular lipid oxidation due to an increase in blood free fatty acid concentration, which can reduce carbohydrate utilization and cause insulin resistance (Randle PJ et al 1963). It was later reported that increasing free fatty acid and lipid oxidation in the blood reduces not only glucose oxidation but also non-oxidative carbohydrate utilization (Lee KU et al 1988). In addition, effective blood sugar control may be required; chronic hyperglycemia can cause diabetic complications, such as myocardial infarction or stroke, through the production of reactive oxygen species (Kim MH et al 2023). Excessive generation of free radicals causes cell damage and death; diabetes is induced by the oxidation of cellular components due to endogenous antioxidant activity (Bashan N et al 2009). Furthermore, increased free fatty acids and leptin in diabetic patients also contribute to the production of reactive oxygen species, thus causing pancreatic beta cell dysfunction leading to insulin secretion disorders (Evans JL et al 2002). Insulin helps transport amino acids through cell membranes, promotes protein synthesis in muscle tissue and liver, and inhibits protein breakdown. If there is a lack of insulin in the body due to diabetes symptoms, protein catabolism is accelerated in muscle tissue and liver, and amino acids in the body are converted to glucose through gluconeogenesis, raising blood sugar levels. As a result, urea synthesis increases in the liver and branch chain amino acids are unable to flow into the muscles, resulting in higher blood levels, and a decrease in body protein in the muscles, leading to weakened resistance and symptoms of body weakness (Guillausseau PJ et al 2008).
Oral hypoglycemic agents are used to improve hyperglycemia in diabetic patients; they promote insulin secretion by acting on the pancreas, blocking hepatic glucose biosynthesis improving insulin sensitivity in peripheral tissues, and acting on the gastrointestinal tract to reduce glucose absorption. Morever, other agents include those that inhibit degradation and absorption as well as those that prevent incretin degredation, thereby promoting insulin secretion (Anonymous 2019; Lee BR & Park PS 2022). Research on diabetes management using food materials has been conducted; this was to decrease adverse side effects caused by drug treatment. Morever, the lipid reduction effect of dietary restriction (Lee BR et al 2001), anti-diabetic property of fermented chaga mushroom (Cha JY et al 2006), and inhibitory capability of silk protein enzyme hydrolysates against increasing blood sugar have been observed in animal experiments (Lee KH et al 2007). However, clinical trials involving humans remain scarce.
Soybean (Glycine max L.) is the main component of soymilk, and contains nutrients such as unsaturated fatty acids, iron, and niacin, as well as various physiologically active substances, such as isoflavones, saponins, phytic acids, phytosterols, and dietary fiber (Kim DK et al 2014). In addition, as it is almost cholesterol-free, awareness of it as a vegetable health drink for preventing chronic degenerative diseases has expanded (Carroll KK & Kurowska EM 1995; Kim DK et al 2014). A previous study (Babashahi M et al 2015) reported that the consumption of vegetable soy milk lowered blood LDL-cholesterol levels; interest in vegetable soy milk is therefore increasing. Recently, to improve the quality of soymilk, the release of functional soymilk containing extracts from black beans, buckwheat sprouts, sweet potatoes, pumpkin, wild hair, black sesame, ginseng, and nuts has increased (Yang H & Zhang L 2009; Kim DK et al 2014).
Therefore, in this study, we chose Sahmyook Foods’ commercially available diabetic soy milk and administered it to elderly diabetic patients as a snack alternative to ensure balanced nutritional intake. This study aimed to evaluate the impact of soymilk consumption on dietary management and blood parameters among elderly individuals with diabetes.
MATERIALS AND METHODS
1. Patient Recruitment
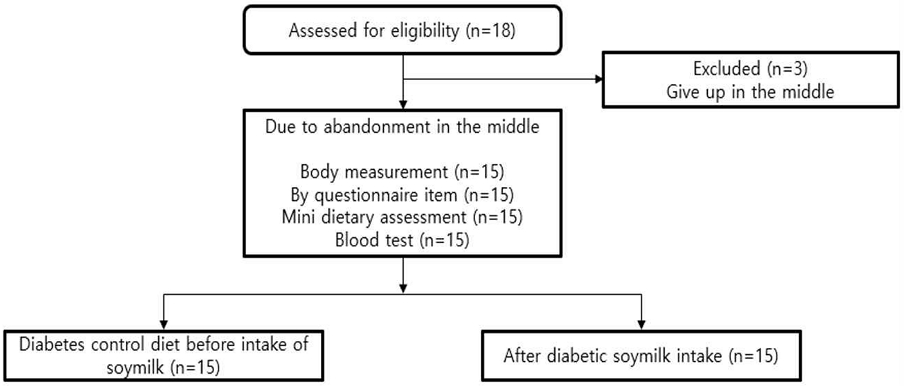
This study enrolled patients who visited and were hospitalized at Sahmyook Medical Center. A type 2 diabetic patient receiving oral blood sugar medication was selected as a test subject due to fatigue, overeating, excessive drinking, polyuria, delayed wound healing, and diabetic HbA1c of 6.5% or higher. To recognize differences according to the diseases and medications administered, selection was made after discussion with the head of the Department of Endocrinology at Sahmyook Medical Center. This study was conducted at the Sahmyook Medical Center from November 2021 to October 2022 with subjects aged 65—80 years old. In this study, the dropout rate was considered as a study to confirm maintenance and improvement through food intake, and the sample size was selected to be around 18 people to calculate statistical values. Among these, 15 people who participated in the experiment until the end were selected (Fig. 1).
Recruitment documents were posted on a bulletin board for patients visiting the Department of Endocrinology at Sahmyook Medical Center. After reading the recruitment notice for the study, a study description and informed consent form were distributed to the those who expressed their intention to participate. After receiving an explanation of the study through consultation with the doctor-in-charge, the participants were allowed to decide whether to participate in the study. Subjects were considered participants of this study upon providing written consent to the researcher or nurse-in-charge. Before participating in this study, subjects were sufficiently informed about the side effects of soymilk consumption, such as abdominal pain and vomiting. In addition, other precautions, and measures for various dropout situations were also explained. The test was conducted and approved by the Sahmyook University Institutional Bioethics Committee SYU 2021-12-006-002, Sahmyook Medical Center 116286-202204-HR(BR)-02.
2. Ingredients and Intake of Soymilk
The diabetes-controlling soy milk selected in this study was developed by Sahmyook Foods (Cheonan, Korea) and is currently available in the market. It is manufactured from undiluted soymilk and black sesame paste. The sugar content was 2% or 200 kcal per pack. It was developed as a snack substitute for diabetic patients by determining its osmolality (280 mOsm/kg H2O) and calorie composition ratio (protein: fat:carbohydrate=16:40:44). Table 1 shows the ingredients and nutritional information of soymilk used for diabetes management. The subjects were allowed to drink soymilk for diabetes control continuously for 4 weeks; the difference before and after drinking was measured through body measurements, health habit surveys, dietary life evaluation surveys, nutrient intake status surveys, and blood analysis. Significance was verified through a statistical analysis using two sets of comparison data. To monitor the patients, a weekly dietary survey was conducted using a smart application. The progress of the clinical trial was checked through frequent phone consultations with the patient. The diabetes control diet soymilk consumption method was used to avoid the intake of other snacks; the intake of 2—3 packs of diabetes control diet soymilk was deemed as a snack substitute per day.
3. Comparison of Body Measurements
Height, weight, and body mass index (BMI) were measured using Inbody 4.0 (Bioimpedence method, Biospace, Seoul, Korea), which uses the impedance principle to measure obesity.
4. Health Habit Survey
Health habits were surveyed only for the respondents of the questionnaire, regardless of sex. The contents of the survey included smoking experience, smoking status, number of cigarettes smoked, drinking experience, drinking status, amount of alcohol consumed, exercise status, and exercise capability.
5. Nutrition Management Diet and Dietary Life Evaluation Survey
Items such as the experience of eating a diabetic tube diet, reason for eating a diabetic control diet, reason for judgment, intention to consume a diabetic control diet, and intention to purchase a diabetic control diet were investigated through a questionnaire.
To diagnose dietary habits, participants were simply evaluated using a 10-item dietary assessment (Kim WY et al 2003). The items of the questionnaire were ‘Milk & its products, daily’, ‘Meat, fish, egg, soybean etc, every meal’, ‘Kimchi, vegetables, every meal’, ‘Fruits & other juices, daily’, ‘Fried foods, more than 2 times per week’, ‘High fat meat, more than 2 times per week’, ‘Add more salt or soy source at meals’, ‘3 meals a day, regularly’, ‘Ice cream, cake, cookies, carbonated drinks as snack, more than 2 times per week’, and ‘Variety of food (balanced diet)’.
6. Investigation of Nutrient Intake Status
A 24-hour recall method was used. Nutrient intake was calculated from the investigated dietary intake data using Can-pro (Computer Aided Nutritional Analysis Program) version 6.0 (2022), which developed by the Korean Society of Nutrition. The nutrient intake status of the survey participants was compared with the calorie requirement estimate, recommended intake (RI), and adequate intake (AI), which are used for evaluating meal intake among males and females aged 65 to 79 years as listed in the Dietary Reference Intakes for Koreans (The Korean Nutrition Society 2020).
7. Blood Analysis
The blood samples of 18 subjects who provided consent were directly collected by a nurse under the supervision of a doctor. The samples were then analyzed as follows.
Approximately 2 mL of collected blood was placed in an EDTA-2K-treated bottle (CBC bottle, Green Cross) for general blood components;, 8-way agitation was performed to prevent coagulation. White blood cell (WBC), red blood cell (RBC), hematocrit (Hct), hemoglobin (Hb), mean cell volume (MCV), mean cell hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelets (PLT), mean platelet volume (MPV), plateletcrit (PCT), and platelet distribution width (PDW) were analyzed using ADVIA 120 (Bayer, NY, USA).
Blood was collected. Subsequently, erythrocyte sedimentation rate (ESR) was measured using ALIFAX test-1 (ALIFAX Co., Polverara, Italy); glucose was measured using an ADAMS glucose GA-1171 analyzer (ARKRAY, Inc., Kyoto, Japan); glycoalbumin was measured using Hitachi 7600 (Hitachi Medical Co., Ltd., Tokyo, Japan); HbA1c was analyzed using an ADAMS HbA1c HA-8180 analyzer.
The serum cholesterol content was measured using the o-phthaldehyde method as described by Cho WK & Choi JH (2007). Samples were aliquoted by 0.1 mL. Then 0.3 mL of 33% KOH solution and 3.0 mL of 95% ethanol were added and mixed thoroughly. The serum was heated in a water bath at 60℃ for 15 minutes and then cooled. Five mL of nucleic acid was then added and mixed. Distilled water (3.0 mL) was added and mixed well for 1 min. The layers were separated to obtain a 1.0 mL nucleic acid layer. The nucleic acid layer was concentrated and dried with nitrogen, and 2.0 mL of o-phthaldehyde reagent was added and mixed well. After 10 minutes, 1.0 mL of concentrated sulfuric acid was added as a coloring reagent and mixed well. After adding sulfuric acid, the absorbance was measured at 550 nm using a spectrophotometer (Human Corporation, Seoul, Korea) within 10—90 min. The cholesterol content was quantified according to a standard calibration curve (Choi KS et al 2016). In addition, by applying the method as described by Kim HJ et al (2009), protein, albumin, glomerular filtration rate (CRP), blood urea nitrogen (BUN), and creatinine levels were simultaneously analyzed using a Hitachi 7020, automatic blood biochemical analyzer.
8. Data Analysis and Statistical Methods
As a study comparing before and after consumption of beverages, the χ2-test (p<0.0001 or p<0.05) was conducted to verify the significance of the health habit survey and dietary life evaluation surveys. After calculating the mean and S.E. of the physical measurement survey, nutrient intake status survey, and blood analysis, significance was verified (p<0.05) by using a paired t-test.
RESULTS
1. Body Measurement
Table 2 presents the general information of the survey participants. The average age of the survey subjects was 73.27±2.31 years. The average height of male participants was 161.88±2.70 cm, while that of female participants was 148.70±1.51 cm. Prior to starting the diabetes control diet, males weighed 56.56±4.85 kg, and females weighed 45.19±2.80 kg. After completing the diabetes control diet, males weighed 52.47±5.83 kg, whereas females weighed 51.10±2.60 kg. Compared to the height standards for individuals aged 65—74 (164.9 cm for males and 152.1 cm for females), both male and female participants in our study were shorter by approximately 3 cm on average. Specifically, following the diabetes-control diet, males tended to lose around 4 kg, whereas females tended to gain about 5 kg. Furthermore, compared to the body weight standards (61.2 kg for males and 49.7 kg for females) specified in the Dietary Reference Intakes for Koreans (KDRI) (The Korean Nutrition Society 2020) for individuals aged 65—74, male participants exhibited a weight reduction of approximately 5—8 kg, while female participants showed lower body weight after completing the diabetes control diet.
The BMIs of male subjects before and after consuming the diabetic control diet were 22.95±1.97 kg/m2 and 19.76±3.12 kg/m2, respectively. For females, the BMIs were 18.88±1.16 kg/m2 before and 20.74±1.06 kg/m2 after diabetic control diet consumption. On average, weight increased by approximately 1 kg after diabetic control diet consumption. According to KDRI (The Korean Nutrition Society 2020) standards, the BMI values were within the normal range of 22.5 kg/m2 for males and 21.5 kg/m2 for females. However, five women had a BMI value of 15.87±0.89 kg/m2, which was lower than the BMI reference value of 18.5 kg/m2. Additionally, during the clinical trial period, BMI levels decreased in male elderly people and increased in female elderly people. In the case of elderly men, calorie intake increased, but the increase in exercise is believed to be the cause of weight loss. In a previous study (Hariri M et al 2015), diabetic patients in their late 50s who consumed soymilk containing probiotics (Lactobacillus planetarium A7) were compared with a placebo group;, the results showed heights of 163.6±6.0 cm and body weights of 70.84±2.41 kg. The BMI was 26.68±0.71 kg/m2; although there was no significant difference, systolic blood pressure was reported to decrease.
2. Health Habits
Table 3 shows the health habits of the respondents who responded to the questionnaire. The experience of smoking decreased from 40.0% in male diabetic patients to 20.0% after taking the diabetes management diet, and it was found that about 10 cigarettes per day bloomed. Male and female diabetic patients’ drinking experience was found to be 10—40%, and male diabetic patients answered that they drink up to 7—9 cups once they drink. The rate of exercise was found to be about three to four times a week for most of the survey subjects. The male elderly reported that they drank about once every 2 days (Choe JS et al 2004). Drinking and smoking are part of the lifestyle of older adults. Drinking habits impair the absorption, transport, and utilization of nutrients, which cause an imbalance in dietary intake, decreasing appetite, and interference with nutrient absorption and metabolism (Park JJ 2017).
3. Dietary Life Evaluation Survey
Dietary evaluations are presented in Table 4. Among elderly male patients, it was found that none of them consumed milk after following the diabetes control diet, whereas 20.0—50.0% of elderly female patients answered occasional milk consumption regardless of adherence to the diabetes control diet. The percentage of elderly male patients who included ‘meat, fish, eggs, beans, tofu, etc.’ in every meal decreased, while the percentage who consumed ‘one fruit or one glass of fruit juice daily’ was 60.0%. Additionally, there was a 60.0% increase in the proportion of elderly males who reported eating three meals a day regularly. Among elderly females, there was a tendency for the percentage of those eating three meals a day regularly to increase after following the diabetes control diet. In particular, tolerance to alcohol decreases with age, and side effects increase (Park JJ 2017). Smoking has been reported to reduce taste sensitivity, serum antioxidant nutrient level, and fruit and vegetable intake (Park JJ 2017). In a previous study (Lee JA & Lee YN 2012), 100% of rural elderly people ate three meals a day, which is higher that of urban elderly people (60.8%);, however, 60.4% of rural elderly people reported having irregular meals. Hong SM & Choi SY (1996) reported that the rate of regularly eating was 74.6% for elderly males and 55.6% for elderly females. In a study by Kim SH (1977), the rate of eating regularly was reported to be 59.5% in the case of the elderly over 60 years of age. In this study, approximately 70% of the elderly patients with diabetes followed a regular diet after consuming a diabetes-control diet. Compared with previous studies (Hong SM & Choi SY 1996; Kim SH 1997), there was no significant change in past and present eating habits.
4. Evaluation of Nutrient Intake Status
The nutrient intake of the survey participants is presented in Table 5. Energy intake before and after diabetes control diet intake tended to increase from 1,190.27±121.00 kcal to 1,403.23±121.83 kcal for males, and significantly increased from 807.42±88.87 kcal to 1,297.61±156.06 kcal for females (p<0.010). Looking at the nutrient intake status in this study, the energy intake of females aged 65 to 74 years was presented as 1,600 kcal in the KDRI (The Korean Nutrition Society 2020). The overall energy intake increased from less than the standard after eating a diabetes control diet. Protein intake increased from 45.51±5.80 g to 66.47±7.88 g in males and from 32.68±5.24 g to 43.72±5.50 g in females before and after intake of the diabetes control diet. The protein intake status was determined to be approximately 45 g per day for female aged 65—74 years as presented in the KDRI (The Korean Nutrition Society 2020). In addition, fat intake also increased to 46.45±7.40 g (p<0.046) and 42.49±6.29 g (p<0.009), after intake of the diabetes control diet for both male and females respectively. Vitamin B12 in diabetic elderly males increased by 2.79-times from 6.29±3.63 mg before taking the diabetes control diet to 17.56±3.96 mg after taking the diabetes control diet (p<0.033). In the diabetic elderly females, vitamins A and E increased by 687.49±196.10 RE (p<0.034) and 12.97±1.61 mg (p<0.023), after diabetic intake, respectively. Vitamins B1 (p<0.019), B2 (p<0.065), B6 (p<0.022), B12 (p<0.015), niacin (p<0.025), C (p<0.039), and folic acid (p<0.010) increased significantly by 1.58—3.67 times after diabetes control diet intake. In the KDRI (The Korean Nutrition Society 2020), the recommended vitamin B6 intake for female aged 65—74 years was 1.4 mg. The results of this study reached the recommended nutrient intake for Koreans after consuming a diabetes-controlled diet. According to the KDRI (The Korean Nutrition Society 2020), the recommended intake for females aged 65—74 is 13 mg for niacin, 100 mg for vitamin C, 2.4 mg for vitamin B12, and 400 μg DFE/d for folic acid.
After intake of diabetes-controlled diet in female elderly, calcium 730.57±180.33 mg (p<0.061), iron 18.60±2.49 g (p<0.012), zinc 9.32±1.39 mg (p<0.036), potassium 3,122.26±381.88 mg (p<0.012), selenium 73.22±12.15 mg (p<0.028), etc., and nutrient intake increased more than twice. Dietary fiber intake in the elderly female also increased significantly from 18.39±4.63 g to 32.45±4.07 g (p<0.012). Calcium intake before and after diabetes control diet increased from 269.63±61.90 mg to 446.65±98.03 mg in males and from 314.73±40.04 mg to 730.57±180.33 mg in females. Iron intake increased by 1.6 and 2 times, respectively, from 10.70±2.62 mg to 17.11±1.73 mg in males and from 9.28±1.75 mg to 18.60±2.49 mg in females before and after diabetes control diet (p<0.05). In elderly people aged 65 years or older residing in Ulsan, calories, protein, vitamins A, B2, and C fell short of the recommended amounts (Hong SM & Choi SY 1996). Calcium intake was found to be low (Hong SM & Choi SY 1996). Elderly people are nutritionally very vulnerable due to physical changes and psychological, social, and economic factors, and are often accompanied by chronic degenerative diseases (Hong SM & Choi SY 1996). Additionally, elderly people have difficulty purchasing, cooking, and eating food. Previous studies (Splett PL 1994) have reported that about half of elderly people in the United States suffer from malnutrition. Malnutrition in the elderly increases functional disability and disease morbidity, and is an important risk factor for frailty, increasing the risk of death in the elderly (Lee SE & Lee Eliza 2018; Wirth R et al 2018). A study targeting the elderly in Korea also found that elderly people with insufficient calorie and protein intake not only had a high overall risk of death, but also a high risk of cardiovascular disease-related mortality, showing that nutritional status is a serious cause of death in the elderly (Kim HR 2016). Looking at the results of nutrient intake in this study, it was confirmed that the nutrient intake of elderly men and women before consuming soy milk was below the standard suggested by KDRI (The Korean Nutrition Society 2020), but that nutrient intake overall improved after consuming soy milk.
In particular, the intake of selenium among minerals increased significantly from 44.37±7.92 mg to 73.22±12.15 mg (p<0.028) in the case of elderly females. Selenium is an essential trace element in the body and a component of selenium-containing proteins involved in antioxidant action, activation of thyroid hormone, and redox of cells (Rayman MP 2000; Woo JH & Lim WS 2017). Recently, interest in selenium has been increasing;, effective in preventing diseases such as aging, cancer, and viral infections (Lee SH et al 2005). According to the KDRI (The Korean Nutrition Society 2020), the sufficient intake of dietary fiber for women aged 65—74 years is 20 g. In this study, dietary fiber intake significantly increased from 18.39±4.63 g to 32.45±4.07 g before and after diabetes control diet intake (p<0.012). Approximately 5—20% of the population complained of constipation. In the elderly, intestinal motility decreases and the incidence of atonic constipation increases (Kim JY et al 2006). Constipation treatment is important in dietary therapy;, sufficient dietary fiber and water intake are important. The ingestion of dietary fiber, a plant component that is not digested in the intestine, is the best method to be used primarily for preventing and treating constipation;, it absorbs water in the large intestine to soften the stool and increase its volume. In addition, it helps in the growth of E. coli and enlarges its transformation, which is helpful in relieving constipation (Lembo A & Camilleri M 2003).
5. Basic Blood Analysis
Basic blood test results are shown in Table 6. In elderly male patients, there was no significant difference observed in basic blood levels before and after intake the diabetes-controlled diet. However, among elderly female patients, significant changes were noted in several blood parameters: WBC (3.47±0.41 103/uL), Hct (31.62±3.62%), Hb (10.33±1.19 g/dL), MCV (82.55±9.41 fL), MCH (27.01±3.12 pg), MCHC (29.40±3.28 g/dL), RDW (11.69±1.33%), PLT (177.70±24.90 103/uL), and PDW (10.91±1.28 fL). Following the consumption of the diabetic control diet, significant changes were observed in the same parameters: WBC (2.31±0.64 103/uL), Hct (21.06±5.79%), Hb (6.90±1.91 g/dL), MCV (54.72±14.93 fL), MCH (17.90±4.89 pg), MCHC (19.61±5.35 g/dL), RDW (7.94±2.17%), PLT (112.60±31.19 103/uL), and PDW (7.35±2.04 fL) (p<0.05). Overall, the WBC count decreased below the normal range in elderly male patients, whereas in elderly female patients, the levels of WBC, Hct, Hb, and MCHC were observed to be lower than the normal range. In particular, the blood levels of Hct, Hb, and MCHC decreased in postmenopausal elderly male, and it is believed that one of the causes of increased risk of anemia is the frequency of consumption of tea and coffee, which interfere with iron absorption when eating food. Additionally, if the clinical trial period continues longer while consuming soymilk consistently, it is believed that the levels of Hct, Hb, and MCHC in the blood can be increased.
6. Diabetes-Related Blood Factors
The diabetes-related blood factors are shown in Table 7. It was observed that ESR tended to decrease, although the difference was not statistically significant. In elderly male patients, there was a significant decrease in blood glucose levels from 156.60±32.36 mg/dL to 134.60±46.80 mg/dL before and after the intake of the diabetes control diet (p<0.05). Similarly, in elderly female patients, the blood glucose level significantly decreased from 147.70±35.49 mg/dL before initiating the diabetes control diet to 98.90±35.18 mg/dL after intake of diabetes control diet (p<0.05). Additionally, HbA1c levels significantly decreased from 7.40±0.45% and 6.86±0.99% in male and female elderly patients, respectively, before the diabetic control diet, to 5.64±1.43% and 4.61±1.36%, respectively, after diabetic control diet intake (p<0.05). A study conducted by Bielefeld D et al (2020) found that incorporating legumes such as chickpeas, peas, lentils, and soybeans into a diet with a low glycemic index led to a decrease in HbA1c levels in the blood by approximately 0.10 to 0.50%. Specifically, soy milk contains various indigestible components such as sucrose, stachyose, araban, galactan, fiber, and starch. Research has shown that when consumed in appropriate amounts, soy oligosaccharides can promote the proliferation of Bifidus bacteria, which are beneficial intestinal bacteria, thereby potentially aiding in bowel regulation (Hayakawa K et al 1990). In this study, blood cholesterol levels significantly decreased from 129.40±13.11 mg/dL to 101.20±26.98 mg/dL in elderly male patients before and after consuming soy milk as a diabetes management diet.
7. Blood-Related Lipids and Proteins
Lipid- and protein-related blood factors are presented in Table 8. Before and after consuming diabetes-controlled soymilk, blood cholesterol levels in elderly male patients ranged from 129.40±13.11 mg/dL to 101.20±26.98 mg/dL, and in elderly female patients, from 136.90±17.46 mg/dL to 96.10±26.62 mg/dL (p<0.05). In a previous study involving elderly females aged 65 to 80 (Hwang HJ et al 2023), no significant change was observed in blood HbA1c levels before and after incorporating soy milk into the diabetes management diet. However, there was a slight decrease in blood cholesterol levels from 144.00±8.02 mg/dL to 142.46±7.49 mg/dL. Additionally, in rat studies, the consumption of soy milk combined with L. plantarum A7 (KC 355240) and cuminum cyminum essential oil led to significant reductions in total cholesterol and LDL in the blood with increasing duration of nutritional intervention compared to the consumption of control or regular soy milk. Furthermore, bioactive ingredients like isoflavones have been reported to exhibit efficacy against diabetes (Babashahi M et al 2020). Protein intake from food was increased through the consumption of soymilk in the diabetes management diet of male and female elderly people, but because the experiment period was short, it is believed that it was not intended to increase blood protein and albumin levels. Following the consumption of diabetes-controlled soymilk, blood BUN levels showed a decrease, ranging from 20.56±4.13 mg/dL to 16.40±5.51 mg/dL in elderly male patients and from 17.76±2.84 mg/dL to 13.64±3.89 mg/dL in elderly female patients (p<0.05). Similarly, in this study, blood BUN levels before and after consuming soy milk into the diabetes management diet demonstrated a decline, ranging from 20.56±4.13 mg/dL to 16.40±5.51 mg/dL in elderly male patients and from 17.76±2.84 mg/dL to 13.64±3.89 mg/dL in elderly female patients (p<0.05). Blood creatine concentrations decreased in elderly male and female after consuming soy milk as a diabetes management diet. A comparison of the serum creatine concentration of soymilk containing L. plantarum A7 and regular soymilk in patients with type 2 diabetic shown that the, creatine concentration was significantly decreased when consuming soymilk containing L. plantarum A7. It has also been reported that the renal function index improves in patients with type 2 diabetes and kidney disease (Abbasi B et al 2017). Oxidative stress causes an electrolyte imbalance due to hormonal abnormalities and decreased kidney function (Manna P et al 2009). BUN, creatinine concentration, and GFR have been used as prognostic indicators of kidney function (Kwon MA et al 2003). Elevated BUN and creatinine concentrations increase the likelihood of developing dyslipidemia such as acute stroke (Lin WC et al 2015; Oh DY et al 2019), atherosclerosis, cerebral infarction (Schrock JW et al 2012), and hypertensive heart disease (Akimoto T et al 2011).
CONCLUSIONS
This study was conducted to help balanced nutritional supply by ingesting soy milk from the diabetes management diet with the consent of elderly diabetics. In the elderly male patients, the proportion of ‘Meat, fish, egg, soybean etc, every mean’ decreased, and in the elderly female, the proportion of ‘3 males a day, regularly’ increased after ingestion of the diabetes management diet. In the elderly female with diabetes, the intake of vitamin (e.g., A, E, B1, B2, B6, B12, Niacin, C) and folate was significantly increased after ingestion of the diabetes management diet. In the elderly female, minerals (e.g., calcium, iron, zinc, potassium, selenium) and dietary fiber were increased after ingestion of the diabetes management diet. Blood glucose levels were significantly reduced from 156.60±32.36 mg/dL to 134.60±46.80 mg/dL in male elderly and from 147.70±35.49 mg/dL to 98.90±35.18 mg/dL in female elderly patients before and after ingestion of the diabetes management diet (p<0.05). In addition, HbA1c levels and cholesterol levels were significantly reduced in male and female elderly patients after ingestion of the diabetes management diet (p<0.05). Summarizing this study, it is judged that consuming soy milk as a diabetes management diet helps alleviate malnutrition and malnutrition in the elderly, and helps lower blood glucose, HbA1c levels, and cholesterol levels.
Acknowledgments
This result was supported by Sahmyook Foods, and a clinical trial was conducted at Sahmyook Medical Center, and we appreciate it.
FUNDING
This result was supported by Sahmyook Foods.
References
- Abbasi B, Ghiasvand R, Mirlohi M (2017) Kidney function improvement by soy milk containing Lactobacillus plantarum A7 in type 2 diabetic patients with nephropathy: A double-blinded randomized controlled trial. Iran J Kidney Dis 11(1): 36-43.
-
Akimoto T, Ito C, Kato M, Ogura M, Muto S, Kusano E (2011) Reduced hydration status characterized by disproportionate elevation of blood urea nitrogen to serum creatinine among the patients with cerebral infarction. Med Hypotheses 77(4): 601-604.
[https://doi.org/10.1016/j.mehy.2011.06.044]

- Anonymous (2019) Drugs for type 2 diabetes. Med Lett Drugs Ther 61(1584): 169-178.
- Babashahi M, Mirlohi M, Ghiasvand R, Azabakht L (2015) Comparison of soymilk and probiotic soymilk effects on serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol in diabetic Wistar rats. ARYA Atheroscler 11(Suppl 1): 88-93.
-
Babashahi M, Mirlohi M, Ghiasvand R, Azadbakht L, Mosharaf L, Torki-Baghbadorani S (2020) Effects of probioticsoy milk fermented by Lactobacillus plantarum A7 (KC 355240) added with Cuminum cyminum essential oil on fasting blood glucose levels, serum lipid profile and body weight in diabetic Wistar rats. Int J Prev Med 11: 88-93.
[https://doi.org/10.4103/ijpvm.IJPVM_541_17]

-
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A (2009) Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89(1): 27-71.
[https://doi.org/10.1152/physrev.00014.2008]

-
Bielefeld D, Grafenauer S, Rangan A (2020) The effects of legume consumption on markers of glycaemic control in individuals with and without diabetes mellitus: A systematic literature review of randomised controlled trials. Nutrients 12(7): 2123.
[https://doi.org/10.3390/nu12072123]

- Carroll KK, Kurowska EM (1995) Soy consumption and cholesterol reduction: Review of animal and human studies. J Nutr 125(3): 594S-597S.
- Cha JY, Bang SJ, Kim JW, Park SH, Lee CH, Cho YS (2006) Hypoglycemic effects of fermented Chaga mushroom (Inonotus obliquus) in the diabetic otsuka long evans to kushima fatty OLETF rats. Food Sci Biotechnol 15(5): 739-745.
-
Chatterjee P, Nagi N, Agarwal A, Das B, Banerjee S, Sarkar S, Gupta N, Gangakhedkar RR (2020) The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J Med Res 151(2 & 3): 147-159.
[https://doi.org/10.4103/ijmr.IJMR_519_20]

- Cho WK, Choi JH (2007) Effect of pyroligneous liquor on lipid metabolism in serum of CD rats. Korean J Nutr 40(1): 24-30.
- Choe JS, Kwon SO, Paik HY (2004) Health-related quality of life by socioeconomic factors and health-related behaviors of the elderly in rural area. Korean J Rural Med 29(1): 29-41.
-
Choi KS, Kim YH, Shin KO (2016) Effect of mulberry extract on the lipid profile and liver function in mice fed a high fat diet. Korean J Food Nutr 29(3): 411-419.
[https://doi.org/10.9799/ksfan.2016.29.3.411]

-
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev 23(5): 599-622.
[https://doi.org/10.1210/er.2001-0039]

-
Guillausseau PJ, Meas T, Virally M, Laloi-Michelin M, Médeau V, Kevorkian JP (2008) Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab 34(Suppl 2): S43-S48.
[https://doi.org/10.1016/S1262-3636(08)73394-9]

- Hariri M, Salehi R, Feizi A, Mirlohi M, Kamali S (2015) The effect of probiotic soy milk and soy milk on anthropometric measures and blood pressure in patients with type II diabetes mellitus: A randomized double-blind clinical trial. ARYA Atheroscler 11(Suppl 1): 74-80.
-
Hayakawa K, Mizutani J, Wada K, Masai T, Yoshihara I, Mitsuoka T (1990) Effects of soybean oligosaccharides on human faecal flora. Microb Ecol Health Dis 3: 293-303.
[https://doi.org/10.3109/08910609009140252]

- Hong SM, Choi SY (1996) A study on meal management and nutrient intake of the elderly. J Korean Soc Food Sci Nutr 25(6): 1055-1061.
-
Hwang HJ, Park SH, Park SY, Ahn SR, Park SJ, Lee YJ, Chon KJ, Song CH, Moon DG, Shin KO (2023) A study on changes in nutritional and blood conditions before and after consumption of diabetic soymilk in elderly women with diabetes. J Korean Soc Food Sci Nutr 52(4): 341-349.
[https://doi.org/10.3746/jkfn.2023.52.4.341]

-
Kim DK, Choi EJ, Kim CH, Kim YB, Kim EM, Kum JS, Park JD (2014) Physicochemical properties of rice grain-added soymilk. J Korean Soc Food Sci Nutr 43(8): 1278-1282.
[https://doi.org/10.3746/jkfn.2014.43.8.1278]

-
Kim HJ, Yang HJ, Kim MH, Ryu GH, Jung JY (2009) Effect of saengmaec-san on the level of blood glucose and serum components in streptozocin-induced diabetic rats. J Korean Soc Food Sci Nutr 38(9): 1179-1186.
[https://doi.org/10.3746/jkfn.2009.38.9.1179]

-
Kim HR (2016) Quality of diet and nutritional intake and mortality risk among South Korean adults based on 12-year follow-up data. Korea J Community Nutr 21(4): 354-365.
[https://doi.org/10.5720/kjcn.2016.21.4.354]

- Kim JY, Kim OH, Yoo HJ, Kim TI, Kim WH, Yoon YD, Lee JH (2006) Effect of fiber supplements on functional constipation. J Korean Nutr 39(1): 35-43.
-
Kim MH, Kim HJ, Heo HJ, An MJ, Hong MR, Jeong HS, Lee JS (2023) Synergistic effects of sorghum extract and metformin on anti-diabetic activities in HepG2 Cells. J Korean Soc Food Sci Nutr 52(1): 17-25.
[https://doi.org/10.3746/jkfn.2023.52.1.17]

- Kim SH (1977) A survey of food habits of the elderly over sixty years of age in Seoul. Korean J Nutr 10(4): 59-67.
- Kim WY, Cho MS, Lee HS (2003) Development and validation of mini dietary assessment index for Koreans. Korean J Nutr 36(1): 83-92.
-
Kwon MA, Kim GS, Hong JK, Jo HS, Kim JK, Yang MK, Lee BD (2003) The effects of 0.45% and 0.9% saline solutions on serum sodium concentrations in chronic renal failure patients. Korean J Anesthesiol 44(4): 462-468.
[https://doi.org/10.4097/kjae.2003.44.4.462]

- Lee BR, Cha JH, Park JY, Bae HY, Koh CN, Park PS (2001) Effect of dietary restraction on the serum lipid level in OLETF rats. J Korean Soc Food Sci Nutr 30(6): 1210-1214.
-
Lee BR, Park PS (2022) Effect of combined treatment with catechin and quercetin on hepatic glucose metabolism in diabetic rats. J Korean Soc Food Sci Nutr 51(1): 12-18.
[https://doi.org/10.3746/jkfn.2022.51.1.12]

-
Lee HA, Yu MN, Kim HJ, Sung JH, Jeong HS, Lee JS (2020) Antioxidant and anti-Diabetic activities of ethanol extracts of cereal grains and legumes. J Korean Soc Food Sci Nutr 49(4): 323-328.
[https://doi.org/10.3746/jkfn.2020.49.4.323]

-
Lee HI, Turkyilmaz A, Lee MK (2023) Anti-hyperglycemic effects of fermented mealworm extract on type 2 diabetic mice. J Korean Soc Food Sci Nutr 52(2): 431-436.
[https://doi.org/10.3746/jkfn.2023.52.4.431]

-
Lee JA, Lee YN (2012) Comparison of healthy life style and chronic disease management between urban and rural older adults. Korean J Rehabil Nurs 15(2): 100-108.
[https://doi.org/10.7587/kjrehn.2012.100]

-
Lee KH, Park JR, Seo JS (2007) Nutritional status of the elderly living in a private silver town of Busan metropolitan city, Korea. J Korean Soc Food Sci Nutr 36(10): 1293-1299.
[https://doi.org/10.3746/jkfn.2007.36.10.1293]

-
Lee KU, Lee HK, Koh CS, Min HK (1988) Artificial induction of intravascular lipolysis by lipidheparin infusion leads to insulin resistance in man. Diabetologia 31(5): 285-290.
[https://doi.org/10.1007/BF00277409]

- Lee SE, Lee Eliza (2018) Effects of nutrition related factors onmortality risk among community-residing older adults in Korea. J Digit Converg 16(10): 343-350.
-
Lee SH, Kwak WS, Kim WY (2005) Studies on the selenium type and metabolism of selenium accumulation in the selenium-enriched mushroom, flammulina velutipes, and its spent mushroom composts. J Anim Sci & Technol 47(2): 305-316.
[https://doi.org/10.5187/JAST.2005.47.2.305]

-
Lembo A, Camilleri M (2003) Chronic constipation. N Engl J Med 349: 1360-1368.
[https://doi.org/10.1056/NEJMra020995]

-
Lin WC, Shih HM, Lin LC (2015) Preliminary prospective study to assess the effect of early blood urea nitrogen/creatinine ratio-based hydration therapy on poststroke infection rate and length of stay in acute schemic stroke. J Stroke Cerebrovasc Dis 24(12): 2720-2727.
[https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.08.002]

-
Manna P, Sinha M, Sil PC (2009) Retraction note: Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids 56(1): 24.
[https://doi.org/10.1007/s00726-024-03390-w]

-
Nassar M, Daoud A, Nso N, Medina L, Ghernautan V, Bhangoo H, Nyein A, Mohamed M, Alqassieh A, Soliman K, Alfishawy M, Sachmechi I, Misra A (2021) Diabetes mellitus and COVID-19: Review article. Diabetes Metab Syndr 15(6): 102268.
[https://doi.org/10.1016/j.dsx.2021.102268]

-
Oh DY, Kang DS, Lee YG, Kim HS (2019) Effects of turmeric (Curcuma longa L.) supplementation on blood urea nitrogen and enzyme activities in dyslipidemic rats. JESI 28(5): 475-483.
[https://doi.org/10.5322/JESI.2019.28.5.475]

- Park JJ (2017) A study on the relationship between lifestyle and health status of smoking and drinking in the elderly. KJS 15(1): 417-428.
-
Randle PJ, Carland PB, Hales CN, Newsholme EA (1963) The glucose fatty acid cycle: Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1(7285): 785-789.
[https://doi.org/10.1016/S0140-6736(63)91500-9]

-
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225): 233-241.
[https://doi.org/10.1016/S0140-6736(00)02490-9]

-
Schrock JW, Glasenapp M, Drogell K (2012) Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin Neurol Neurosurg 114(7): 881-884.
[https://doi.org/10.1016/j.clineuro.2012.01.031]

-
Splett PL (1994) Federal food assistance programs: A step to food security for many. Nutrition Today 29(2): 6-13.
[https://doi.org/10.1097/00017285-199403000-00002]

-
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ (2022) IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183: 109119.
[https://doi.org/10.1016/j.diabres.2021.109119]

- The Korean Nutrition Society (2020) Dietary Reference Intakes for Koreans. The Korean Nutrition Society, Seoul, Korea. pp ⅸ-ⅹⅷ.
-
Wirth R, Pourhassan M, Streicher M, Hiesmay M, Schindler K, Sieber CC, Volkert (2018) The impact of dysphagia on mortality of nursing home residents: Results from the nutritionDay project. J Am Med Dir Assoc 19(9): 775-778.
[https://doi.org/10.1016/j.jamda.2018.03.016]

-
Woo JH, Lim WS (2017) Anticancer effect of selenium. Ewha Med J 40(1): 17-21.
[https://doi.org/10.12771/emj.2017.40.1.17]

-
Yang H, Zhang L (2009) Changes in some components of soymilk during fermentation with the basidiomycete Ganoderma lucidum. Food Chem 112(1): 1-5.
[https://doi.org/10.1016/j.foodchem.2008.05.024]