Antioxidant Activity of the Inclusion Complexes of Catechin, Epicatechin, Morin Hydrate, and Quercetin with Hydroxypropyl-β-Cyclodextrin
Abstract
This study aimed to formulate inclusion complexes of hydroxypropyl-β-cyclodextrin (HP-β-CD) with four flavonoids, namely, catechin (CA), epicatechin (EC), morin hydrate (MH), and quercetin (QC) and evaluated their antioxidant activities through in vitro assays. The antioxidant potential was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC), oxygen radical absorbance capacity (ORAC), and hydrogen peroxide, hydroxyl, and superoxide radical scavenging assays. The antioxidant activity varied across the complexes and assays, largely influenced by the flavonoid structure, particularly the number and position of hydroxyl groups and their orientation within the cyclodextrin cavity. EC/HP-β-CD showed the highest DPPH activity (101.57 mM TE/g), while MH/HP-β-CD was most effective in the ABTS, FRAP, CUPRAC, and hydrogen peroxide assays, indicating strong reducing capacity. QC/HP-β-CD exhibited the highest hydroxyl and superoxide radical scavenging activity, highlighting its ability to neutralize reactive oxygen species. The HP-β-CD complexation improved the antioxidant performance of flavonoids by stabilizing reactive groups and enhancing solubility. These results emphasize the importance of the molecular structure in determining antioxidant efficiency and indicate that HP-β-CD inclusion complexes are promising candidates for antioxidant therapies and prevention of oxidative stress-related disorders.
Keywords:
flavonoids, hydroxypropyl-β-cyclodextrin (HP-β-CD), inclusion complexes, antioxidant activityINTRODUCTION
Medicinal plants have long played a crucial role in various branches of healthcare, including allopathic medicine, herbal remedies, homeopathy, and aromatherapy, serving as a primary source of numerous modern pharmaceuticals (Mikawlrawng K et al 2017; Pellow J & Nienhuis C 2018). In particular, plant extracts containing flavonoids are widely used as therapeutic agents due to their affordability and accessibility, especially in developing countries (Sádaba LM et al 2008). Flavonoids, a diverse group of plant-derived polyphenols commonly present in the human diet, have garnered significant attention owing to their potent antioxidant properties, which are considered stronger than those of vitamins C and E (Mladěnka P et al 2011). Among naturally occurring flavonoids, catechin (CA), epicatechin (EC), morin hydrate (MH), and quercetin (QC) are well-recognized for their biological activities (Yang SL et al 2019). CA and EC have been widely applied in food and nutraceutical products due to their efficacy in treating inflammatory conditions, cancer, and other diseases, as well as in the formulation of anti-aging skincare products (Maurya PK & Rizvi SI 2009; Nagarajan S et al 2008). MH demonstrates strong antioxidant activity and metal ion chelation, alongside a wide range of biological effects, including anti-mutagenic, anti-inflammatory, anti-neoplastic, cardioprotective, and anticancer properties, as well as inhibition of xanthine oxidase, protein kinase C, and cell proliferation (Hanasaki Y et al 1994; Yu Z et al 2006). Also, QC has attracted considerable interest for its antioxidant activity, inhibition of carcinogen-activating enzymes, modulation of signal transduction pathways, and interactions with receptors and other cellular proteins (Murakami A et al 2008). Despite their pharmacological potential, the clinical application of CA, EC, MH, and QC is significantly limited by their poor water solubility, low stability, and limited oral bioavailability, which hinder their therapeutic efficacy (Zhao Y et al 2009). Various strategies have been employed to address these issues, among which the formation of inclusion complexes with β-cyclodextrins (β-CDs) is one of the most promising approaches (Yang SL et al 2019).
β-CD is a cyclic oligosaccharide composed of seven glucose units. Its unique molecular structure, featuring a hydrophobic internal cavity and a hydrophilic external surface, allows it to encapsulate a variety of guest molecules, thereby enhancing their stability, solubility, and bioavailability, while also offering selective molecular orientation and protection (Stepniak A et al 2015). Hydroxypropyl-β-cyclodextrin (HP-β-CD), a hydroxyalkylated derivative of β-CD, possesses improved water solubility, low toxicity, and strong inclusion capability (Gould S & Scott RC 2005). Owing to these advantages, HP-β-CD has been extensively utilized to enhance the pharmaceutical properties of active pharmaceutical ingredients (APIs), and is considered more effective than native β-CD for various applications (Garnero C et al 2010; Pérez-Abril M et al 2017). As the first β-CD derivative approved by the U.S. Food and Drug Administration (FDA), HP-β-CD is used in the food and agriculture industries (Yuan C et al 2008) and has been widely investigated as a drug delivery vehicle (Liu HK et al 2010). Additionally, HP-β-CD has proven effective in improving the solubility of poorly water-soluble drugs (Liu J et al 2006). Therefore, the objective of this study was to prepare inclusion complexes of HP-β-CD with four flavonoids—catechin (CA), epicatechin (EC), morin hydrate (MH), and quercetin (QC)—and to evaluate their antioxidant activities. The antioxidant potential of the inclusion complexes was assessed through DPPH, ABTS, FRAP, CUPRAC, ORAC, and hydrogen peroxide, hydroxyl, and superoxide radical scavenging assays.
MATERIALS AND METHODS
1. Materials and Reagents
Hydroxypropyl-β-cyclodextrin (HP-β-CD), catechin (CA), epicatechin (EC), morin hydrate (MH), quercetin (QC), ascorbic acid (Vit. C), 6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid (Trolox), 2, 2-diphenyl-1-picrylhydrazyl (DPPH), 2, 2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2, 2-azobis(2-methylpropionamidine) dihydrochloide (AAPH), fluorescein, copper(II) chloride (CuCl2·2H2O), neocuproine (Nc), ammonium acetate (NH4Ac), potassium dihydrogen phosphate (KH2PO4), sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O), iron(III) chloride hexahydrate (FeCl3·6H2O), 2, 4, 6-tripyridyl-s-triazine (TPTZ), p-nitrosodimethylaniline (p-NDA), nitroblue tetrazolium (NBT), hydrogen peroxide, methanol, hydrochloric acid (HCl), and chloroform were purchased from Sigma-Aldrich.
2. Preparation of Flavonoid/HP-β-CD Inclusion Complexes
The inclusion complexes were prepared following the freeze-drying method described by Pralhad T & Rajendrakumar K (2004), using a 1:1 molar ratio of flavonoids to HP-β-CD. Specifically, 4 mmol of HP-β-CD and 4 mmol of each flavonoid were dissolved in 50 mL of distilled water. The mixture was incubated at 30℃ with shaking at 150 rpm for 24 hours, then filtered through a 0.45 μm membrane filter. The resulting solution was frozen at —80℃ and lyophilized using a freeze dryer (FDU-1200, EYELA, Tokyo, Japan) for 24 hours to obtain a powdered complex.
3. DPPH Radical Scavenging Activity
The DPPH radical scavenging activity of the inclusion complexes was evaluated using the method of Delgado-Andrade C et al (2005). A 200 μL aliquot of each complex was added to 1 mL of DPPH solution (74 mg/L in methanol). The DPPH solution, prepared fresh daily, had an initial absorbance of 1.8 at 520 nm. After mixing, the solution was shaken for 30 minutes, and absorbance was measured at 520 nm. Trolox solutions (0.05—0.5 mM) were used to generate a standard calibration curve. All measurements were performed in quintuplicate, and results were expressed as mM Trolox equivalents (TE) per gram of sample.
4. ABTS Radical Scavenging Activity
The ABTS radical scavenging capacity was assessed according to the method of Re R et al (1999). ABTS radical cation (ABTS⁺·) was generated by reacting a 7 mM ABTS stock solution with 2.45 mM potassium persulfate (K2S2O8), followed by dilution with 5 mM PBS (pH 7.4) to achieve an absorbance of 0.70 ± 0.02 at 730 nm. To 3 mL of the ABTS⁺· solution, 50 μL of each sample or Trolox standard was added. Absorbance was recorded at 730 nm for 20 minutes using a spectrophotometer (Synergy HTX spectrophotometer, Biotech Instruments, Winooski, VT, USA). Trolox standards were used for calibration. All measurements were conducted five times, and results were expressed as mM TE/g of sample.
5. Ferric Reducing Antioxidant Power
The ferric-reducing ability was evaluated according to the method of Benzie IFF & Strain JJ (1996). A reaction mixture consisting of 900 μL of FRAP reagent, 90 μL of distilled water, and 50 μL of each sample was prepared. The FRAP reagent was freshly prepared by mixing 2.5 mL of 10 mM TPTZ in 40 mM HCl, 2.5 mL of 20 mM FeCl3·6H2O, and 25 mL of 0.3 M acetate buffer (pH 3.6). The absorbance was monitored every 15 seconds for 30 minutes at 595 nm and 37℃ using a spectrophotometer (Synergy HTX spectrophotometer, Biotech Instruments, Winooski, VT, USA). Trolox solutions were used for calibration. Each sample was measured five times, and results were expressed as mM TE/g of sample.
6. Oxygen Radical Absorbance Capacity Activity
The ORAC assay was conducted as described by Huang D et al (2002b). A 153 mM solution of 2,2-azobis(2-methylpropionamidine) dihydrochloide (AAPH) was prepared fresh daily in 75 mM phosphate buffer (pH 7.4). A fluorescein stock solution (4 × 10—3 mM) was diluted 1:1,000 in the same buffer. In a microplate, 150 μL of the fluorescein working solution was added to each interior well, followed by 25 μL of phosphate buffer (blank), Trolox standard, or sample. Plates were equilibrated at 37℃ for 30 minutes. The reaction was initiated by adding 25 μL of AAPH using a microplate reader injector. Fluorescence was measured every minute for 90 minutes at an excitation of 485 nm and emission of 520 nm. Each sample was analyzed five times. ORAC values were calculated based on area under the curve (AUC) as follows:
Where R1 is the fluorescence reading at the initiation of the reaction and Rn is the last measurement.
7. Cupric Reducing Antioxidant Capacity Activity
The cupric reducing antioxidant capacity activity of the inclusion complexes was investigated according to the procedures reported by Karadirek Ş et al (2016). The solutions of 10 mM CuCl2·2H2O and 1 M ammonium acetate were prepared in pure distilled water. Neocuproine solution at 7.5 mM concentration was daily prepared in absolute ethanol. After mixing 1 mL Cu(II), 1 mL neocuproine and 1 mL ammonium acetate solutions, 0.5 mL of samples filtrates was added to this mixture, and the final volume was completed to 4.1 mL with pure distilled water. Then, the solution was let to stand for 30 min to achieve equilibrium. Absorbance measurement of the resulting cuprous-neocuproine complex was performed at 450 nm against a reagent blank prepared from 1 mL Cu(II), 1 mL neocuproine and 1 mL ammonium acetate solutions and 1.1 mL pure distilled water. All experiments were repeated five times.
8. Hydrogen Peroxide Radical Scavenging Activity
Hydrogen peroxide scavenging activity was measured spectrophotometrically based on the method by Jayaprakasha GK et al (2004). One milliliter of various concentrations of the samples in methanol was added to 2 mL of hydrogen peroxide (20 mM) in phosphate-buffered saline (PBS). After incubation for 10 minutes, absorbance was measured at 230 nm using a spectrophotometer (Synergy HTX spectrophotometer, Biotech Instruments, Winooski, VT, USA). All experiments were repeated five times.
9. Hydroxyl Radical Scavenging Activity by p-NDA
Hydroxyl radical scavenging activity was determined using the p-nitrosodimethylaniline (p-NDA) assay described by Srinivasan R et al (2007). A reaction mixture containing 0.5 mL of each sample in DMSO was mixed with 0.5 mL each of 0.1 mM ferric chloride, 0.1 mM EDTA, 0.1 mM ascorbic acid, 2 mM hydrogen peroxide, and 0.01 mM p-NDA in 20 mM phosphate buffer (pH 7.4). The final volume was 3 mL. Absorbance was measured at 440 nm. All samples were measured five times.
10. Superoxide Radical Scavenging Activity by Alkaline DMSO
Superoxide radicals were generated by adding sodium hydroxide to air-saturated DMSO, which reduces nitroblue tetrazolium (NBT) to a formazan dye measurable at 560 nm. The reaction mixture contained 1 mL of alkaline DMSO (5 mM NaOH in 0.1 mL water), 0.1 mL of NBT (1 mg/mL), and 0.3 mL of sample in DMSO, totaling 1.4 mL. After mixing, absorbance was recorded at 560 nm (Srinivasan R et al 2007). All samples were analyzed five times.
11. Statistical Analysis
The experimental data were subjected to analysis of variance (ANOVA). Significant differences between the mean values, as determined from measurements carried out in five replicate tests (i.e., p<0.05), were obtained by Duncan’s multiple-range test using statistical analysis software (SPSS 20.0, IBM Inc., NY, USA).
RESULTS AND DISCUSSION
1. DPPH Radical Scavenging Activity
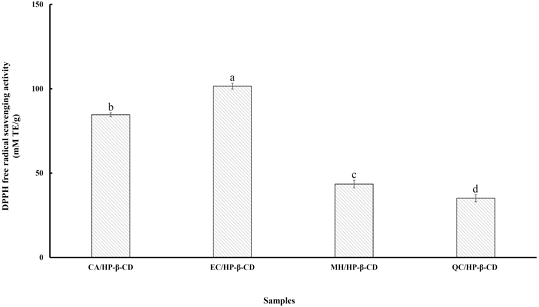
In the DPPH assay, antioxidants reduce the stable DPPH radical to the yellow-colored compound diphenylpicrylhydrazine. This method relies on the ability of hydrogen-donating antioxidants to convert DPPH into its non-radical form, DPPH-H, in an alcoholic solution. The DPPH assay primarily operates through a single electron transfer (SET) mechanism, with hydrogen atom transfer (HAT) playing a minor role. Additionally, the DPPH radical is more stable than both superoxide and hydroxyl radicals (Siriwardhana SKW & Shahidi F 2002). The DPPH radical scavenging activity of flavonoid/HP-β-CD inclusion complexes are shown in Fig. 1. The values ranged from 35.13 to 101.57 mM Trolox equivalents (TE) per gram of complex. Among the tested complexes, EC/HP-β-CD showed significantly higher DPPH scavenging activity (p<0.05) than the others, with the highest value of 101.57 mM TE/g, followed by CA/HP-β-CD, MH/HP-β-CD, and QC/HP-β-CD. According to Cai YZ et al (2006), the antioxidant activity of polyphenols is influenced by the number and position of hydroxyl groups, with ortho-dihydroxy groups and glycosylation playing key roles. Xie PJ et al (2015) also reported that increased hydroxyl groups enhance antioxidant potential. Furthermore, Benavente-García O & Castillo J (2008) highlighted that structural features—such as the 3′,4′-dihydroxy B-ring configuration, 2,3-double bond with a 4-oxo group, and hydroxyls at positions 3 and 5—are crucial for activity. Anselmi C et al (2008) further suggested that the antioxidant activity of polyphenols encapsulated in cyclodextrins depends on the orientation of hydroxyl groups. If these functional groups are buried within the cyclodextrin cavity, their antioxidant potential is reduced in proportion to the strength of the interaction. However, if the hydroxyl groups remain exposed outside the cavity, their antioxidant activity remains unaffected. Therefore, the encapsulation of polyphenols with HP-β-CD can influence their antioxidant effectiveness based on the structure of the polyphenol, the number and position of hydroxyl groups, and how those groups are oriented within the cyclodextrin cavity.
2. ABTS Radical Scavenging Activity
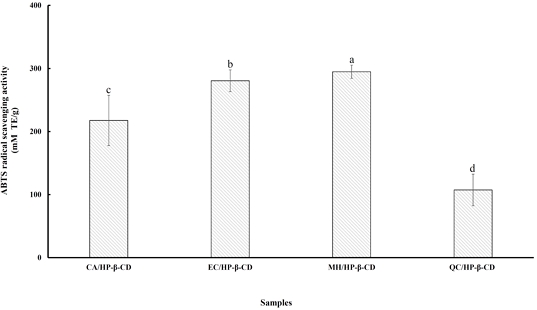
The ABTS radical scavenging assay is a relatively recent method that uses a more reactive, chemically generated radical. It is particularly suitable for evaluating complex antioxidant mixtures such as plant extracts, beverages, and biological fluids. Due to its solubility in both organic and aqueous media and its stability across a wide pH range, ABTS·⁺ is widely used to estimate antioxidant activity (Nenadis N et al 2004). Fig. 2 shows the ABTS radical scavenging capacities of flavonoid/HP-β-CD inclusion complexes. The ABTS values were 294.79 mM TE/g for MH/HP-β-CD, 280.48 mM TE/g for EC/HP-β-CD, 217.56 mM TE/g for CA/HP-β-CD, and 107.36 mM TE/g for QC/HP-β-CD. Among them, MH/HP-β-CD showed the highest activity, followed by EC/HP-β-CD, CA/HP-β-CD, and QC/HP-β-CD (p<0.05). The enhanced antioxidant activity of these complexes may be attributed to the protection cyclodextrins provide against rapid oxidation (Mercader-Ros MT et al 2010), partly due to increased solubility in biological systems (Haiyun D et al 2003). Inclusion within the cyclodextrin cavity can also stabilize the phenoxyl radicals formed during reaction with ABTS·⁺, thereby delaying their further oxidation. Additionally, cyclodextrins can act as secondary antioxidants, further enhancing antioxidant effects (López-Nicolás JM et al 2007). These findings suggest that cyclodextrins not only extend the half-life of antioxidant compounds but also broaden their applications. The high activity of flavonoids is likely due to hydroxyl groups, which serve as electron-donating substituents. Replacing these groups significantly reduces antioxidant potential. Furthermore, both the number and position of hydroxyl and methoxy groups, as well as steric effects near phenolic OH groups, influence activity (Amorati R et al 2007). Therefore, HP-β-CD can serve as a secondary antioxidant and enhance the efficacy of flavonoids.
3. Ferric Reducing Antioxidant Power
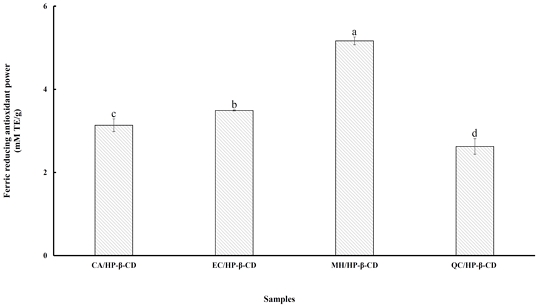
The FRAP (Ferric Reducing Antioxidant Power) assay evaluates the antioxidant’s reducing ability by measuring its reaction with the ferric tripyridyltriazine (Fe3⁺-TPTZ) complex, resulting in the formation of a blue-colored ferrous (Fe²⁺) complex (Benzie IFF & Strain JJ 1996). Antioxidants act as reducing agents by donating hydrogen atoms to the ferric complex, thereby disrupting the radical chain reaction. Higher absorbance corresponds to greater antioxidant activity, as reflected in a higher FRAP value. Fig. 3 presents the FRAP values of flavonoid/HP-β-CD inclusion complexes. Among them, the MH/HP-β-CD complex exhibited the highest ferric reducing power (5.16 mM TE/g), significantly higher (p<0.05) than the other complexes, with values ranging from 2.62 to 5.16 mM TE/g. This trend is consistent with the results observed in the ABTS assay. According to Jullian C et al (2010), differences in antioxidant activity among cyclodextrin complexes may result from changes in the redox behavior of phenol groups and/or stabilization of radicals within the cyclodextrin cavity. In addition to improving the solubility of flavonoids, cyclodextrins may enhance their antioxidant properties. Alvarez-Parrilla E et al (2005) further noted that the reducing capacity of polyphenols depends on factors such as the number and conjugation of hydroxyl groups. For flavonols, strong antioxidant activity is associated with (a) 3′,4′- dihydroxy substitutions, (b) a 2,3-double bond conjugated with a 4-oxo group in the C-ring, and (c) hydroxyl groups at positions 3 and 5. Therefore, these findings suggest that complexation with HP-β-CD can effectively improve the reducing power and overall antioxidant potential of flavonoids.
4. Oxygen Radical Absorbance Capacity Activity
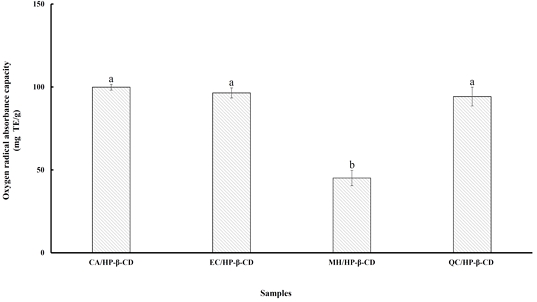
The ORAC_FL(fluorescein) assay provides more reliable results for antioxidant capacity, as it measures the inhibition of peroxyl radical-induced oxidation of fluorescein, initiated by the thermal decomposition of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH). Unlike other assays, ORAC_FL uniquely accounts for both the extent and duration of inhibition, combining them into a single ORAC value. This value is widely used to standardize the antioxidant activity of foods (Mantegna S et al 2012). Fig. 4 shows the ORAC values of flavonoid/HP-β-CD inclusion complexes, which ranged from 45.09 to 99.87 mg TE/g. The CA/HP-β-CD complex exhibited the highest ORAC value, followed by EC/HP-β-CD, QC/HP-β-CD, and MH/HP-β-CD. Interestingly, except for MH/HP-β-CD, there were no significant differences among the other complexes. Similar findings were reported by Folch-Cano C et al (2010), who observed consistent ORAC results when polyphenols were complexed with HP-β-CD. Lucas-Abellán C et al (2011) also noted that ORAC is particularly suitable for evaluating the antioxidant activity of polyphenol-CD complexes, as fluorescein and AAPH are not affected by cyclodextrin encapsulation. Therefore, these findings are associated with previous studies, confirming the reliability of ORAC_FL in assessing antioxidant activity in cyclodextrin-complexed polyphenols, due to its resistance to interference from encapsulation components.
5. Cupric Reducing Antioxidant Capacity Activity
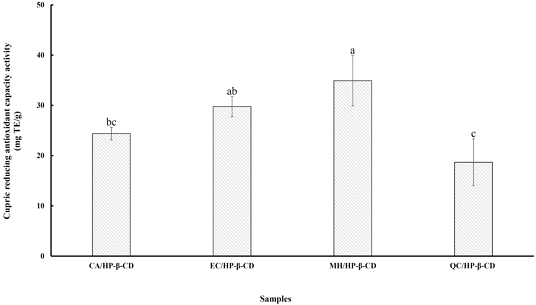
The cupric reducing antioxidant capacity (CUPRAC) assay evaluates the total antioxidant capacity of both hydrophilic and hydrophobic samples by measuring their ability to reduce Cu2⁺ to Cu⁺ in the presence of chelating agents such as neocuproine, bathocuproine, and bathocuproinedisulfonic acid disodium salt (Rubio CP et al 2016). This method has been widely applied to human serum, food products, and plant extracts (Apak R et al 2005). Fig. 5 shows the CUPRAC values for flavonoid/HP-β-CD inclusion complexes. The antioxidant capacities were ranked as follows: MH/HP-β-CD (34.90 mg TE/g) > EC/HP-β-CD (29.75 mg TE/g) > CA/HP-β-CD (24.38 mg TE/g) > QC/HP-β-CD (18.67 mg TE/g). The MH/HP-β-CD complex exhibited significantly higher antioxidant activity (p<0.05) than the others, consistent with the results of the ABTS and FRAP assays. The CUPRAC reagent oxidizes phenolic hydroxyl groups to their corresponding quinones, making the total antioxidant capacity of a polyphenol roughly proportional to the number and position of Ar—OH groups. The efficiency of electron transfer also depends on the molecule’s overall conjugation (Apak R et al 2004). Additionally, flavonoids with similar conjugation and hydroxyl group counts show enhanced antioxidant potency when an ortho-dihydroxy group is present in the B-ring (Huang D et al 2002a). Hydroxycinnamic acids, due to resonance stabilization via the —CH=CH—COOH moiety, generally demonstrate greater antioxidant capacity than hydroxybenzoic acids, even with similar substitution patterns (Cai YZ et al 2006). Therefore, these findings highlight the influence of polyphenol structure—particularly the number and position of hydroxyl groups and molecular conjugation—on antioxidant performance. The results also suggest that cyclodextrin encapsulation can enhance antioxidant activity by preserving functional groups essential for electron transfer, reinforcing the potential of HP-β-CD complexes in improving the stability and effectiveness of flavonoid-based antioxidants.
6. Hydrogen Peroxide Radical Scavenging Activity
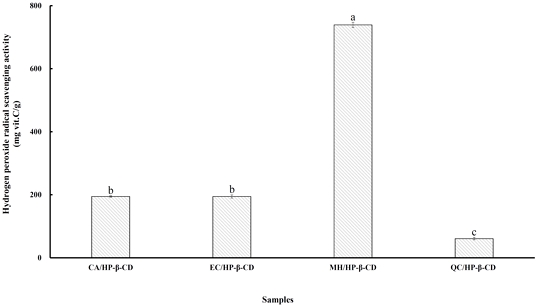
Hydrogen peroxide is a reactive oxygen species that can be naturally produced within biological systems. In vivo, it is generated by several oxidizing enzymes, including superoxide dismutase. Although hydrogen peroxide is not highly reactive on its own, it can cross cellular membranes and slowly oxidize various biomolecules. It also plays a role in the respiratory burst of activated phagocytes (MacDonald-Wicks LK et al 2006). However, hydrogen peroxide can become toxic, as it may lead to the formation of highly reactive hydroxyl radicals within cells (Halliwell B 1991). As such, oxidative stress—resulting from an imbalance between pro-oxidants and antioxidants—has been increasingly recognized as a contributing factor in chronic diseases such as inflammation, cardiovascular disorders, hypertension, and certain types of cancer (Oh TY et al 2001). Therefore, the efficient removal of hydrogen peroxide is essential for maintaining cellular antioxidant defense. Polyphenols have demonstrated the ability to protect mammalian cells from hydrogen peroxide-induced damage, particularly those containing ortho-hydroxy groups, such as quercetin, gallic acid, caffeic acid, and catechin (Nakayama T 1994). Fig. 6 presents the hydrogen peroxide scavenging capacity of flavonoid/HP-β-CD inclusion complexes, compared to ascorbic acid as a reference. The scavenging values ranged from 60.84 to 739.06 mg vitamin C equivalent per gram of complex, following the order: MH/HP-β-CD > EC/HP-β-CD > CA/HP-β-CD > QC/HP-β-CD. This trend is consistent with the results of ABTS, FRAP, and CUPRAC assays. The MH/HP-β-CD complex showed significantly higher hydrogen peroxide scavenging activity (p<0.05) than the other complexes, confirming its strong antioxidant potential. Notably, the scavenging capacity of MH/HP-β-CD was approximately 12 times greater than that of QC/HP-β-CD, which had the lowest value. Therefore, these findings indicate that the ability of MH/HP-β-CD to neutralize hydrogen peroxide highlights its potential as a promising candidate for antioxidant-based therapeutic or preventive applications.
7. Hydroxyl Radical Scavenging Activity by p-NDA
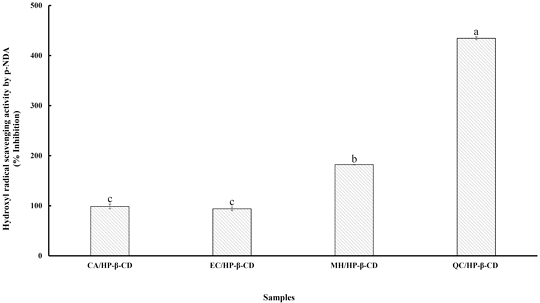
Among the various oxygen radicals, the hydroxyl radical is the most reactive and can cause significant damage to nearby biomolecules (Sakanaka S et al 2005). In this study, the hydroxyl radical scavenging activity of polyphenol/HP-β-CD inclusion complexes was evaluated using the p-NDA bleaching method. In this assay, hydroxyl radicals are generated via the Fenton reaction, where an iron-EDTA complex reacts with hydrogen peroxide in the presence of ascorbic acid. These hydroxyl radicals specifically bleach p-NDA, and the degree of inhibition of this bleaching indicates the scavenging activity of the test compounds (Kumar RS et al 2012). Fig. 7 presents the hydroxyl radical scavenging capacity of flavonoid/HP-β-CD inclusion complexes, with values ranging from 93.91% to 434% relative to the inclusion complexes. The QC/HP-β-CD complex showed significantly higher (p<0.05) scavenging activity than the other complexes. Specifically, the scavenging capacity of QC/HP-β-CD was 4.4, 4.6, and 2.4 times greater than those of CA/HP-β-CD, EC/HP-β-CD, and MH/HP-β-CD, respectively. Quercetin’s superior activity is attributed to its unique structural features. While quercetin shares the same number and positions of hydroxyl groups as catechin, it also contains a 2,3-double bond in the C ring and a 4-oxo group, which enhance electron delocalization. According to Rice-Evans CA et al (1996), three key structural features contribute to effective radical scavenging: An ortho-dihydroxy structure in the B ring, which stabilizes the radical form and facilitates electron delocalization; A 2,3-double bond conjugated with a 4-oxo function in the C ring, which allows electron delocalization from the B ring, enhancing antioxidant activity; The presence of 3- and 5-hydroxyl groups in combination with a 4-oxo function in the A and C rings, which further contribute to radical scavenging capacity. These features help stabilize the resulting phenoxyl radicals through resonance within the aromatic ring system, making quercetin-based complexes particularly effective in neutralizing hydroxyl radicals. Therefore, these findings highlight the critical role of specific structural elements in determining the antioxidant potency of polyphenol compounds. Consequently, the QC/HP-β-CD complex shows strong potential as an effective hydroxyl radical scavenger, emphasizing its value in antioxidant defense systems.
8. Superoxide Radical Scavenging Activity by Alkaline DMSO
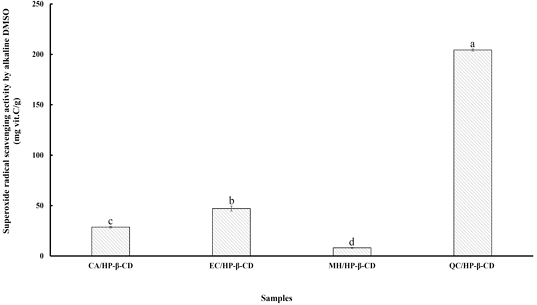
Superoxide is an oxygen-centered radical characterized by selective reactivity. Although it is a relatively weak oxidizing agent with limited intrinsic reactivity, superoxide can give rise to more deleterious reactive oxygen species (ROS), including singlet oxygen and hydroxyl radicals, which are implicated in lipid peroxidation and subsequent cellular damage (Halliwell B & Chirico S 1993). These species are generated through a variety of enzymatic systems. Furthermore, superoxide has been shown to reduce iron complexes such as cytochrome c, thereby acting as a precursor to more reactive radicals capable of interacting with biological macromolecules and contributing to oxidative stress-related tissue injury (Halliwell B & Gutteridge JM 1984). It is also capable of initiating lipid peroxidation directly. Previous studies have reported that the antioxidant properties of certain flavonoids are primarily mediated through their superoxide anion scavenging capacity (Yen GC & Duh PD 1994). Since superoxide radicals are typically the initial ROS formed, their presence can propagate oxidative cascades by generating additional reactive species (Liu F et al 1997). The superoxide radical scavenging activity of flavonoid/HP-β-CD inclusion complexes are presented in Fig. 8, with results expressed as milligrams of vitamin C equivalent per gram of complex. The scavenging capacities ranged from 8.16 to 204.29 mg vitamin C/g. Among the tested complexes, QC/HP-β-CD exhibited the highest activity, which was significantly greater (p<0.05) than that of the other samples. The order of scavenging efficacy was as follows: QC/HP-β-CD (204.29 mg vit. C/g) > EC/HP-β-CD (47.07 mg vit. C/g) > CA/HP-β-CD (28.69 mg vit. C/g) > MH/HP-β-CD (8.16 mg vit. C/g). All inclusion complexes demonstrated measurable superoxide radical scavenging activity. However, the markedly superior performance of the QC/HP-β-CD complex highlights its potential as an effective antioxidant, particularly through mechanisms involving superoxide neutralization. Therefore, these findings suggest that, due to superoxide’s role as a key initiator of oxidative stress and a precursor to more reactive oxygen species, the strong scavenging activity of the QC/HP-β-CD complex makes it a particularly promising antioxidant for reducing oxidative damage in biological systems.
CONCLUSION
This study demonstrated that the inclusion of flavonoids—catechin (CA), epicatechin (EC), morin hydrate (MH), and quercetin (QC)—into HP-β-CD significantly influenced their antioxidant activities, as evaluated through multiple in vitro assays. The antioxidant performance of each complex varied depending on the specific assay and structural characteristics of the flavonoids, including the number and position of hydroxyl groups and the degree of conjugation. Among the tested complexes, MH/HP-β-CD exhibited consistently higher activity in electron transfer-based assays, including ABTS, FRAP, CUPRAC, and hydrogen peroxide scavenging, indicating its strong potential as a reducing agent. In contrast, QC/HP-β-CD demonstrated the most potent scavenging activity against hydroxyl and superoxide radicals, underscoring its effectiveness in neutralizing highly reactive oxygen species. Overall, these results suggest that HP-β-CD encapsulation enhances the antioxidant functionality of flavonoids by stabilizing reactive molecular structures and preserving key functional groups critical for radical scavenging. Therefore, HP-β-CD-based polyphenol inclusion complexes show promise as effective antioxidant agents for therapeutic or preventive applications targeting oxidative stress-related disorders.
Acknowledgments
This work was supported by Kyungnam University Foundation Grant, 2024.
References
-
Alvarez-Parrilla E, Rosa LADL, Torres-Rivas F, Rodrigo-Garcia J, González-Aguilar GA (2005) Complexation of apple antioxidants: Chlorogenic acid, quercetin and rutin by β-Cyclodextrin (β-CD). J Incl Phenom Macrocycl Chem 53: 121-129.
[https://doi.org/10.1007/s10847-005-1620-z]

-
Amorati R, Cavalli A, Fumo MG, Masetti M, Menichetti S, Pagliuca C, Pedulli GF, Viglianisi C (2007) Kinetic and thermochemical study of the antioxidant activity of sulfur-containing analogues of vitamin E. Chemistry 13(29): 8223-8230.
[https://doi.org/10.1002/chem.200700309]

-
Anselmi C, Centini M, Maggiore M, Gaggelli N, Andreassi M, Buonocore A, Beretta G, Facino RM (2008) Non-covalent inclusion of ferulic acid with α-cyclodextrin improves photo-stability and delivery: NMR and modeling studies. J Pharm Biomed Anal 46(4): 645-652.
[https://doi.org/10.1016/j.jpba.2007.11.037]

-
Apak R, Güçlü K, Ozyürek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52(26): 7970-7981.
[https://doi.org/10.1021/jf048741x]

-
Apak R, Güçlü K, Özyürek M, Karademir SE, Altun M (2005) Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic Res 39(9): 949-961.
[https://doi.org/10.1080/10715760500210145]

-
Benavente-García O, Castillo J (2008) Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem 56(15): 6185-6205.
[https://doi.org/10.1021/jf8006568]

-
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 239(1): 70-76.
[https://doi.org/10.1006/abio.1996.0292]

-
Cai YZ, Sun M, Xing J, Luo Q, Corke H (2006) Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci 78(25): 2872-2888.
[https://doi.org/10.1016/j.lfs.2005.11.004]

-
Delgado-Andrade C, Rufián-Henares JA, Morales FJ (2005) Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J Agric Food Chem 53(20): 7832-7836.
[https://doi.org/10.1021/jf0512353]

-
Folch-Cano C, Jullian C, Speisky H, Olea-Azar C (2010) Antioxidant activity of inclusion complexes of tea catechins with β-cyclodextrins by ORAC assays. Food Res Int 43(8): 2039-2044.
[https://doi.org/10.1016/j.foodres.2010.06.006]

-
Garnero C, Zoppi A, Genovese D, Longhi M (2010) Studies on trimethoprim:hydroxypropyl-β-cyclodextrin: Aggregate and complex formation. Carbohydr Res 345(17): 2550-2556.
[https://doi.org/10.1016/j.carres.2010.08.018]

-
Gould S, Scott RC (2005) 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem Toxicol 43(10): 1451-1459.
[https://doi.org/10.1016/j.fct.2005.03.007]

-
Haiyun D, Jianbin C, Guomei Z, Shaomin S, Jinhao P (2003) Preparation and spectral investigation on inclusion complex of β-cyclodextrin with rutin. Spectrochim Acta A Mol Biomol Spectrosc 59(14): 3421-3429.
[https://doi.org/10.1016/S1386-1425(03)00176-8]

-
Halliwell B (1991) Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med 91(3): S14-S22.
[https://doi.org/10.1016/0002-9343(91)90279-7]

-
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57(5): 715S-725S.
[https://doi.org/10.1093/ajcn/57.5.715S]

-
Halliwell B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219(1): 1-14.
[https://doi.org/10.1042/bj2190001]

-
Hanasaki Y, Ogawa S, Fukui S (1994) The Correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med 16(6): 845-850.
[https://doi.org/10.1016/0891-5849(94)90202-X]

-
Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Deemer EK (2002a) Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J Agric Food Chem 50(7): 1815-1821.
[https://doi.org/10.1021/jf0113732]

-
Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL (2002b) High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem 50(16): 4437-4444.
[https://doi.org/10.1021/jf0201529]

-
Jayaprakasha GK, Rao LJ, Sakariah KK (2004) Antioxidant activities of flavidin in different in vitro model systems. Bioorg Med Chem 12(19): 5141-5146.
[https://doi.org/10.1016/j.bmc.2004.07.028]

-
Jullian C, Cifuentes C, Alfaro M, Miranda S, Barriga G, Olea-Azar C (2010) Spectroscopic characterization of the inclusion complexes of luteolin with native and derivatized β-cyclodextrin. Bioorg Med Chem 18(14): 5025-5031.
[https://doi.org/10.1016/j.bmc.2010.05.079]

-
Karadirek Ş, Kanmaz N, Balta Z, Demirçivi P, Üzer A, Hızal J, Apak R (2016) Determination of total antioxidant capacity of humic acids using CUPRAC, Folin-Ciocalteu, noble metal nanoparticle- and solid-liquid extraction-based methods. Talanta 153: 120-129.
[https://doi.org/10.1016/j.talanta.2016.03.006]

-
Kumar RS, Rajkapoor B, Perumal P (2012) Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac J Trop Biomed 2(4): 256-261.
[https://doi.org/10.1016/S2221-1691(12)60019-7]

-
Liu F, Ooi VE, Chang ST (1997) Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci 60(10): 763-771.
[https://doi.org/10.1016/S0024-3205(97)00004-0]

-
Liu HK, Lo YK, Tsai TR, Cham TM (2010) Physicochemical characterizations of ostholehydroxypropyl-β- cyclodextrin inclusion complexes with high-pressure homogenization method. J Food Drug Anal 18(6): 391-397.
[https://doi.org/10.38212/2224-6614.2245]

-
Liu J, Qiu L, Gao J, Jin Y (2006) Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-β-cyclodextrin. Int J Pharm 312(1-2): 137-143.
[https://doi.org/10.1016/j.ijpharm.2006.01.011]

-
López-Nicolás JM, Pérez-López AJ, Carbonell-Barrachina Á, García-Carmona F (2007) Use of natural and modified cyclodextrins as inhibiting agents of peach juice enzymatic browning. J Agric Food Chem 55(13): 5312-5319.
[https://doi.org/10.1021/jf070499h]

-
Lucas-Abellán C, Mercader-Ros MT, Zafrilla MP, Gabaldón JA, Núñez-Delicado E (2011) Comparative study of different methods to measure antioxidant activity of resveratrol in the presence of cyclodextrins. Food Chem Toxicol 49(6): 1255-1260.
[https://doi.org/10.1016/j.fct.2011.03.004]

-
MacDonald-Wicks LK, Wood LG, Garg ML (2006) Methodology for the determination of biological antioxidant capacity in vitro: a review. J Sci Food Agric 86(13): 2046-2056.
[https://doi.org/10.1002/jsfa.2603]

-
Mantegna S, Binello A, Boffa L, Giorgis M, Cena C, Cravotto G (2012) A one-pot ultrasound-assisted water extraction/cyclodextrin encapsulation of resveratrol from polygonum cuspidatum. Food Chem 130(3): 746-750.
[https://doi.org/10.1016/j.foodchem.2011.07.038]

-
Maurya PK, Rizvi SI (2009) Protective role of tea catechins on erythrocytes subjected to oxidative stress during human aging. Nat Prod Res 23(12): 1072-1079.
[https://doi.org/10.1080/14786410802267643]

-
Mercader-Ros MT, Lucas-Abellán C, Fortea MI, Gabaldón JA, Núñez-Delicado E (2010) Effect of HP-β-cyclodextrins complexation on the antioxidant activity of flavonols. Food chem 118(3): 769-773.
[https://doi.org/10.1016/j.foodchem.2009.05.061]

-
Mikawlrawng K, Rani R, Kumar S, Bhardwaj AR, Prakash G (2018) Anti-paralytic medicinal plants - Review. J Tradit Complement Med 8(1): 4-10.
[https://doi.org/10.1016/j.jtcme.2017.02.001]

-
Mladěnka P, Macáková K, Filipský T, Zatloukalová L, Jahodář L, Bovicelli P, Silvestri IP, Hrdina R, Saso L (2011) In vitro analysis of iron chelating activity of flavonoids. J Inorg Biochem 105(5): 693-701.
[https://doi.org/10.1016/j.jinorgbio.2011.02.003]

-
Murakami A, Ashida H, Terao J (2008) Multitargeted cancer prevention by quercetin. Cancer Lett 269(2): 315-325.
[https://doi.org/10.1016/j.canlet.2008.03.046]

-
Nagarajan S, Nagarajan R, Braunhut SJ, Bruno F, McIntosh D, Samuelson L, Kumar J (2008) Biocatalytically oligomerized epicatechin with potent and specific anti-proliferative activity for human breast cancer cells. Molecules 13(11): 2704-2716.
[https://doi.org/10.3390/molecules13112704]

- Nakayama T (1994) Suppression of hydroperoxide-induced cytotoxicity by polyphenols. Cancer Res 54(7 Suppl): 1991s-1993s.
-
Nenadis N, Wang LF, Tsimidou M, Zhang HY (2004) Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J Agric Food Chem 52(15): 4669-4674.
[https://doi.org/10.1021/jf0400056]

-
Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB (2001) Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut 49(3): 364-371.
[https://doi.org/10.1136/gut.49.3.364]

-
Pellow J, Nienhuis C (2018) Medicinal plants for primary dysmenorrhoea: A systematic review. Complement Ther Med 37: 13-26.
[https://doi.org/10.1016/j.ctim.2018.01.001]

-
Pérez-Abril M, Lucas-Abellán C, Castillo-Sánchez J, Pérez-Sánchez H, Cerón-Carrasco JP, Fortea I, Gabaldón JA, Núñez-Delicado E (2017) Systematic investigation and molecular modelling of complexation between several groups of flavonoids and HP-β-cyclodextrins. J Funct Foods 36: 122-131.
[https://doi.org/10.1016/j.jff.2017.06.052]

-
Pralhad T, Rajendrakumar K (2004) Study of freeze-dried quercetin-cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J Pharm Biomed Anal 34(2): 333-339.
[https://doi.org/10.1016/S0731-7085(03)00529-6]

-
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9-10): 1231-1237.
[https://doi.org/10.1016/S0891-5849(98)00315-3]

-
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20(7): 933-956.
[https://doi.org/10.1016/0891-5849(95)02227-9]

-
Rubio CP, Tvarijonaviciute A, Martinez-Subiela S, Hernández-Ruiz J, Cerón JJ (2016) Validation of an automated assay for the measurement of cupric reducing antioxidant capacity in serum of dogs. BMC Vet Res 12(1): 137.
[https://doi.org/10.1186/s12917-016-0760-2]

-
Sádaba LM, Fernández-Robredo P, Rodríguez JA, García-Layana A (2008) Antioxidant effects of vitamins C and E, multivitamin-mineral complex and flavonoids in a model of retinal oxidative stress: The ApoE-deficient mouse. Exp Eye Res 86(3): 470-479.
[https://doi.org/10.1016/j.exer.2007.11.020]

-
Sakanaka S, Tachibana Y, Okada Y (2005) Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem 89(4): 569-575.
[https://doi.org/10.1016/j.foodchem.2004.03.013]

-
Siriwardhana SSKW, Shahidi F (2002) Antiradical activity of extracts of almond and its by-products. J Am Oil Chem Soc 79(9): 903-908.
[https://doi.org/10.1007/s11746-002-0577-4]

-
Srinivasan R, Chandrasekar MJN, Nanjan MJ, Suresh B (2007) Antioxidant activity of Caesalpinia digyna root. J Ethnopharmacol 113(2): 284-291.
[https://doi.org/10.1016/j.jep.2007.06.006]

-
Stepniak A, Belica-Pacha S, Rozalska S, Dlugonski J, Urbaniak P, Palecz B (2015) Study on a host-guest interaction of β-cyclodextrin with tebuconazole in water. J Mol Liq 211: 288-293.
[https://doi.org/10.1016/j.molliq.2015.07.023]

-
Xie PJ, Huang LX, Zhang CH, Zhang YL (2015) Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure-activity relationships. J Funct Foods 16: 460-471.
[https://doi.org/10.1016/j.jff.2015.05.005]

-
Yang SL, Zhao LJ, Chi SM, Du JJ, Ruan Q, Xiao PL, Zhao Y (2019) Inclusion complexes of flavonoids with propylenediamine modified β-cyclodextrin:Preparation, characterization and antioxidant. J Mol Struct 1183: 118-125.
[https://doi.org/10.1016/j.molstruc.2019.01.046]

-
Yen GC, Duh PD (1994) Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem 42(3): 629-632.
[https://doi.org/10.1021/jf00039a005]

-
Yu Z, Fong WP, Cheng CHK (2006) The dual actions of morin (3,5,7,2′,4′-pentahydroxyflavone) as a hypouricemic agent: Uricosuric effect and xanthine oxidase inhibitory activity. J Pharmacol Exp Ther 316(1): 169-175.
[https://doi.org/10.1124/jpet.105.092684]

-
Yuan C, Jin Z, Li X (2008) Evaluation of complex forming ability of hydroxypropyl-β-cyclodextrins. Food Chem 106(1): 50-55.
[https://doi.org/10.1016/j.foodchem.2007.05.045]

-
Zhao Y, Gu J, Yang YC, Zhu HY, Huang R, Jing B (2009) Molecular selective binding of aliphatic oligopeptides by bridged bis(β-cyclodextrin)s with aromatic diamine linkers. J Mol Struct 930(1-3): 72-77.
[https://doi.org/10.1016/j.molstruc.2009.04.040]