
Effects of Solvents with Different Polarities on the Antioxidant Activities of the Leaves and Roots of Allium hookeri
Abstract
The aim of this study was to investigate the effects of solvents with different polarities on the antioxidant activities of leaves and roots of Allium hookeri (A. hookeri). Polyphenolic compounds were extracted using 10 solvents with different polarities; solvent compositions were determined using a simplex-centroid design with ethyl acetate, ethanol, and dichloromethane (extracts I–X). A ternary mixture (polarity index [PI]=3.89) of ethyl acetate, ethanol and dichloromethane (1/3:1/3:1/3, v/v/v) and pure ethanol (PI=5.20) were both deemed efficient for the extraction of total phenolic compounds. The total flavonoid content was highest for leaf and root extracts prepared using a binary mixture of ethyl acetate and ethanol (1/2:1/2, v/v; PI=4.30) and pure dichloromethane (PI=3.70), respectively. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical- scavenging activity was highest (p<0.05) for leaf extract II (PI=5.20) and root extract VI (PI=3.55). In general, the DPPH radical-scavenging activities of leaf extracts were higher than those of root extracts except that of extract VI. The ferric reducing ability was highest (p<0.05) for leaf extract II (PI=5.20) and root extract IX (PI=4.32). Furthermore, the total antioxidant activity was highest (p<0.05) for extract V (PI=3.95) of both leaves and roots and was higher for the leaf extract than the root extract. As a wide range of phytochemicals were recovered from solvents with different polarities, the use of an appropriate extraction solvent is imperative when using A. hookeri extracts as nutraceuticals.
Keywords:
A. hookeri, simplex-centroid design, antioxidant activityINTRODUCTION
The Allium genus belongs to the larger Alliaceae family comprising more than 700 species. Allium species have been used as flavoring agents for thousands of years, and several studies have evaluated their chemical characteristics and therapeutic effects (Block E et al 1992; Rose P et al 2006). Garlic (Allium sativum L.) and onion (Allium cepa L.) have been widely studied and known for their diverse biological properties, including antioxidant, antihypertensive, and anti-inflammatory effects (Jensen GS et al 2008). A member of the Allium genus, Allium hookeri (A. hookeri), is primarily cultivated in southeastern Asian countries such as India, Sri Lanka, Myanmar, Bhutan, and China. It is known for its sweet, bitter, and spicy flavor and thus referred to as “three-tastes namul” in Korea. Its roots and leaves are used in salads, soups, and kimchi owing to its unique flavor (You BR et al 2013; Rhyu DY et al 2013). The beneficial effects of A. hookeri have been associated with a variety of phenols (ferulic acid, gallic acid, and cinnamic acid), phytosterols, linoleic acid, and organosulfur compounds (Ayam VS 2011). A. hookeri root has various functional characteristics such as antioxidant, antimicrobial anti-inflammatory, and antidiabetic properties (Lee KW et al 2014; Lee HJ et al 2014). In general the extraction and purification of phytochemicals and antioxidants from plant materials are affected by various factors such as time, temperature, solvent concentration, and solvent polarity. Depending on the chemical nature, various phytochemicals are extracted using solvents of different polarities, as no single solvent may be reliable for the extraction of all phytochemicals and antioxidant compounds present in the plant material (Lapornik B et al 2005; Iloki-Assanga SB et al 2015). The serial exhaustive extraction method involves successive extraction with solvents of increasing polarity from non-polar (n-hexane) to more polar solvents (water) to ensure the extraction of a wide range of compounds with different polarities (Das K et al 2010; Bimakr M et al 2011; Abdel-Aal EI et al 2015). Studies indicate that solvent polarity significantly affects the extract yield and antioxidant activity of phenolic compounds in plant materials (Ghasemzadeh A et al 2011; Barchan A et al 2014; Ghasemzadeh A et al 2015).
Plant phenolics are more soluble in organic solvents such as methanol, ethanol, and aqueous acetone solutions; however, the diversity of phenolics present in plant tissues may pose challenges during the standardization of the extraction methods. One such possible method involves the selection of a mixture of extracting solvents by changing the solvent proportions within the system (binary, ternary, or even multicomponent mixtures) using experimental mixture designs such as a simplex centroid. The Simplex-centroid is an economic and time-saving method, unlike trial-and-error methods, because it takes the advantage of statistical criteria and minimizes both the model error and number of required experiments (Soares PK et al 2009; Abdullah N & Chin NL 2010). In addition, it is possible to observe synergistic/antagonistic effects with this system that are attributed to the different compounds extracted (Rasera GB et al 2019). From this perspective, the solvent selectivity triangle (also known as Snyder’s triangle) is very useful for choosing the best-suited solvent for extraction (Nyiredy S 2004). Snyder’s triangle classifies solvents according to the solvent strength parameter (P), also called “polarity,” as per the gas-liquid partition data for several test solutes in 81 liquids. The value of the partition coefficients is further used to calculate polarity (P) and selectivity (Xί) (Barwick V 1997). Selectivity here considers all types of solvent-solute interactions except for dispersive interactions (interactions with test solutes that are acidic, basic, or dipolar). Xe is the ability to behave as a proton acceptor, Xd is the ability to behave as a proton donor, and Xn denotes the ability to behave as a strong dipole. In addition, critical extraction parameters include solvent, time, solid-to-solvent ratio, number of extractions, temperature, and partial size of the sample material (Cos P et al 2006). The selection of the extraction solvent depends on the specific nature of the bioactive compound being targeted. The extraction yield and, consequently, the biological activity of vegetal extracts may be strongly affected by the solvent used in the process (Waszkowiak K et al 2015). Different solvents, such as organic and/or aqueous systems, have been reported for the extraction of bioactive compounds (Anwar F & Przybylski R 2012). Recent studies by Quezada N & Cherian G (2012) show that the amount of total phenolic compounds (TPCs) extracted from flaxseeds was affected by solvent polarity; for instance, solvents with low polarities, such as ethyl acetate, are less efficient than more polar solvents. Therefore, in this study, a simplex centroid mixture design of 10 components was used to investigate the effects of solvents with different polarities on the antioxidant activities of A. hookeri leaves and roots.
MATERIALS AND METHODS
1. Chemicals and Reagents
Ethyl acetate, ethanol, dichloromethane, Folin-Ciocalteu’s phenol reagent, sodium carbonate, aluminum chloride, garlic acid, quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-tripyridyl-s-triazine (TPTZ), and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals used were of analytical grade and obtained from Merck (Darmstadt, Germany), unless otherwise stated.
2. Plant Materials
A. hookeri was purchased from Samchaenara Co. (Hadong-gun, Gyeongsangnamdo, Korea). A. hookeri was washed with water to remove any contaminants and divided into roots and leaves, which were freeze-dried (FDU-1200, EYELA, Tokyo, Japan). The materials were ground into a fine powder (60 mesh particle size) using an electric grinder (HMF-3000S, Hanil Electric Co, Seoul, Korea), which was stored at —20℃ prior to use.
3. Preparation of Extracts by Solvents with Different Polarities
To investigate the effect of different polarity solvents on the antioxidant activity of A. hookeri leaves and roots, a simplex-centroid design was used (Fig. 1). Extraction solvents were mixtures of ethyl acetate, ethanol, and dichloromethane in various proportions according to a simplex centroid design. The three points at the vertices of the triangle correspond to extractions carried out using pure solvents, ethyl acetate (Ⅰ), ethanol (Ⅱ), and dichloromethane (Ⅲ). The three midpoints of the sides of the triangle correspond to 1:1 binary mixtures of these solvents. Ternary mixtures using different proportions were also investigated. Each solvent was selected using Snyder’s solvent selectivity triangle, since solvents from different groups in the triangle have different selectivity characteristics (Snyder LR et al 2004). Each extract was prepared by weighing 3.0 g of A. hookeri samples and adding 60 mL of the solvent mixtures listed in Table 1. Each mixture was placed at 4℃ for 24 h and filtered through Whatman #2 and #5 filter paper (Whatman International Limited, Kent, England). The filtrates were evaporated using a vacuum evaporator, freeze-dried, and stored at —75℃ until use. The lyophilized extracts were re-dissolved in each solvent to a concentration of 0.6 mg mL—1.
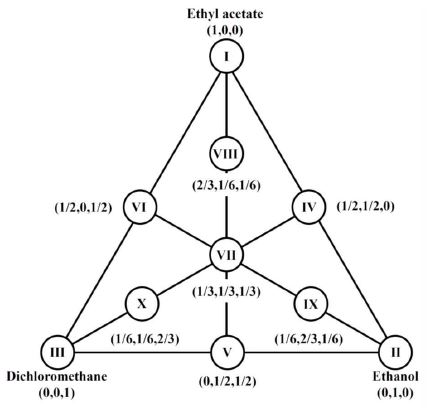
Simplex-centroid design with three axial mixture points to investigate the influence of the different solvent proportions on extract preparation.
4. Determination of Total Phenolic Content
Total phenolic content was measured using Folin-Ciocalteu’s method (Singleton VL et al 1999) with some modifications. Briefly, 0.5 mL of samples and 2.5 mL Folin-Ciocalteu’s phenol reagent were reacted for 5 min at room temperature. Then, 2 mL of 75 g/L sodium carbonate was added to the sample and reacted at room temperature for 2 h. The absorbance value was determined at 760 nm using a SynergyTM HTX spectrophotometer (Biotech® Instruments, Winooski, VT, USA). Ethanol was used as the blank. To construct the standard curve, garlic acid equivalents were used.
5. Determination of Total Flavonoid Content
Total flavonoid content was measured using the Dowd method (Arvouet-Grand A et al 1994) with some modifications. Briefly, 1.5 mL of 2% aluminum chloride and 0.5 mL of sample diluted by 10-fold were mixed and reacted at room temperature for 10 min. The absorbance of the samples was measured at 415 nm. Quercetin was used for the standard curve.
6. DPPH Radical-Scavenging Activity
The antiradical activity of the different samples was estimated according to the procedure described by Delgado-Andrade C et al (2005) with some modifications. A 200 μL aliquot of sample was added to 1 mL of DPPH• (74 mg/L in methanol). The DPPH• solution was prepared daily until a final absorption of 1.8 AU at 520 nm was obtained. The mixture was shaken for 1 h, and the absorption was measured at 520 nm using a Synergy HTX spectrophotometer (Biotech Instruments, Winooski, VT, USA). The temperature in the measurement chamber was set to 30℃. The antiradical activity of the sample is expressed as the percentage of disappearance of the initial purple color. The higher the disappearance, the greater the antiradical activity. Aqueous solutions of Trolox at various concentrations were used for calibration (0.15, 1.0, and 15 mM).
7. Determination of Total Antioxidant Capacity using the Trolox Equivalent Antioxidant Capacity (TEAC) Assay
The antioxidant capacity was estimated according to the procedure described by Delgado-Andrade C et al (2005) with some modifications. Briefly, ABTS+• was produced by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate in the dark at room temperature for 12∼16 h before use. The ABTS+• solution (stable for two days) was diluted with 5 mM phosphate buffered saline (pH 7.4) to an absorbance of 0.70±0.02 at 730 nm. After the addition of 10 μL sample, Trolox standards were added to 4 mL of diluted ABTS+• solution, and the absorbance was read at 20 min using a spectrophotometer (Synergy HTX; Biotech Instruments). Calibration was performed as described for the Trolox stock solution.
8. Ferric Reducing Antioxidant Power Assay
The ferric reducing ability was estimated according to the procedure described by Delgado-Andrade C et al (2005) with some modifications. FRAP reagent (900 μL), prepared freshly and warmed at 37℃, was mixed with 90 μL of distilled water and 30 μL of the sample or water (reagent blank). The final dilution of the test sample in the reaction mixture was 1:34. The FRAP reagent contained 2.5 mL of 10 mM TPTZ solution in 40 mM HCl plus 2.5 mL of 20 mM FeCl3·H2O and 25 mL of 0.3 M acetate buffer, pH 3.6. Readings at the absorption maximum (595 nm) were taken every 15 s using a spectrophotometer (Synergy HTX; Biotech Instruments). The temperature was maintained at 37℃, and the reaction was monitored for up to 30 min. Trolox stock solutions were used to construct the calibration curves.
9. Statistical Analysis
The experimental data were subjected to analysis of variance (ANOVA). Significant differences between the mean values, as determined from measurements carried out in five replicate tests (i.e., p<0.05), were obtained by Duncan’s multiple-range test using statistical analysis software (SPSS 20.0, IBM Inc., NY, USA).
RESULTS AND DISCUSSION
1. TPC and TFC of A. hookeri Extracts Obtained from Solvents with Different Polarities
Phenolic compounds have been reported to exhibit beneficial properties, including antioxidant, anti-inflammatory, and antihypertensive activities, in human and animal studies (Larrosa M et al 2010; Valente LMM et al 2010). In the present study, we used 10 solvents (I-X) to extract bioactive materials from A. hookeri and determined their TPC and TFC levels. As shown in Table 2 and 3, the TPCs ranged from 16.07±0.03 to 48.86±1.04 mg gallic acid equivalent (GAE)/100 g in leaves and 5.93±0.01 to 46.28±0.13 mg GAE/100 g in roots. The TPCs in leaves increased in the order of VII > II > V > I > X > III > VIII > VI > IV > IX, while those in roots increased in the order of II > IV > IX > V > VIII > III > X > VII > I > VI. A ternary mixture (polarity index [PI]=3.89) of ethyl acetate, ethanol, and dichloromethane (1/3:1/3:1/3, v/v/v) was deemed efficient for the extraction of TPCs from A. hookeri leaves, while pure ethanol as a solvent (PI=5.20) was found to be effective for the extraction of TPCs from roots. The difference in TPC levels of leaves and roots was statistically significant (p<0.05) based on the different extraction solvents used. TPCs were unaffected by PI. The TFCs ranged from 22.77±0.16 to 42.53±0.43 mg quercetin equivalent (QE)/100 g in leaves and 13.66±0.23 to 38.11±1.61 mg QE/100 g in roots. The TFCs in leaves and roots increased in the order of IV > III > VI > I > VIII > VII > IX > V > II > X and III > VI > V > VII > IV > VIII > X > IX > II > I, respectively. The TFCs in leaves significantly differed (p<0.05) with all extraction solvents except I, III, and VI. On the other hand, the TFCs in roots significantly differed (p<0.05) only with solvents I, III, V, VI, and VII. The highest level of TFCs was found in extracts prepared using a binary mixture (PI=4.30) of ethyl acetate and ethanol (1/2:1/2, v/v) and dichloromethane as a pure solvent (PI=3.70) for leaves and roots, respectively. TFC was unaffected by PI, as reported for TPC. These results indicated the variation in the type and quantity of phenolic compounds extracted by different solvents. In previous studies, the extraction of phenolic compounds was affected by changes in solvent polarity, extraction conditions (vapor pressure, ratio, time extraction, temperature), and viscosity (Fernández-Agulló A et al 2013). Aqueous mixtures of organic solvents are more efficient for the extraction of phenolic compounds than pure solvents (Fernández-Agulló A et al 2013). However, Thoo YY et al (2010) reported higher extraction efficiency for phenolic compounds using lower concentrations of ethanol, which reflects the sensitivity of TPC to solvents of different polarities. Plants contain different phenolic compounds that exhibit antioxidant activities but differ in polarities. Therefore, the choice of a suitable solvent depends on the type of plant, the part of the plant to be extracted, the sensitivity of the bioactive compounds, and the polarity of the solvent.
2. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical-Scavenging Activity
A compound with antioxidant properties is capable of retarding or preventing the oxidation of other molecules (Moon JK & Shibamoto T 2009). These molecules prevent mutations in macromolecules by quenching reactive oxygen species (ROS) and decreasing ROS-induced oxidative damage. Plant phenols and flavonoids exert strong antioxidative effects and their bioactivities are largely dependent on their phenol and flavonoid content (You S et al 2018). The antioxidant activities of A. hookeri extracts, obtained using solvents of different polarities, were determined using the DPPH assay, and the results are presented in Fig. 2. The DPPH radical-scavenging activity ranged from 0.78±0.01 to 1.83±0.03 mM Trolox equivalent (TE) for leaves and 0.22±0.01 to 1.50±0.03 mM TE for roots. The antioxidant activities of leaf extracts were higher than those of root extracts, excluding the activity of extract VI. The DPPH radical-scavenging activity of leaf and root extracts was in the order of II > IV > V > IX > VIII > VI > I=X > III > VII and VI > V > IX > I > X > II = VII > III > VIII > IV, respectively. Among all leaf extracts, extract II (PI=5.20) showed the highest DPPH radical-scavenging activity (p<0.05), whereas extract VII showed the lowest activity. On the other hand, the DPPH radical-scavenging activity of root extract VI (PI=3.55) was highest (p<0.05), whereas that of root extract IV was lowest. Moreover, the DPPH radical-scavenging activities of leaf extracts were generally higher than those of root extracts, with an exception observed for extract VI. Previous studies have shown that methanol and ethanol can dissolve polar compounds, such as sugars, amino acids, and glycoside compounds (Houghton PJ & Raman A 1998); phenolic compounds with low and medium molecular weights and medium polarity (Yu Lin H et al 2009); aglycone flavonoids (Dehkharghanian M et al 2010); and anthocyanins, terpenoids, saponins, tannins, xantoxilins, totarol, quacinoids, lactones, flavones, phenones, and polyphenols (Cowan MM 1999). Ethyl acetate is an effective solvent for extracting alkaloids, aglycones, glycosidic compounds (Houghton PJ & Raman A 1998), sterols, terpenoids, and flavonoids (Cowan MM 1999). Another study reports that a methanolic extract exhibited higher phenolic content than a dichloromethane extract, which had high flavonoid and alkaloid levels (Baloch R et al 2019). Dehkharghanian M et al (2010) also reported that the differences in the polarities of solvents may determine the variations in the types, compositions, and antioxidant activities of phytochemicals. Therefore, these results indicated that extract II (PI=5.20) and extract VI (PI=3.55) exhibited higher DPPH values than other extracts from the leaves and roots, respectively. The different antioxidant activities of phenolic extracts may be attributed to different extraction solvents, as this property relies on the type and polarity of the extracting solvent.
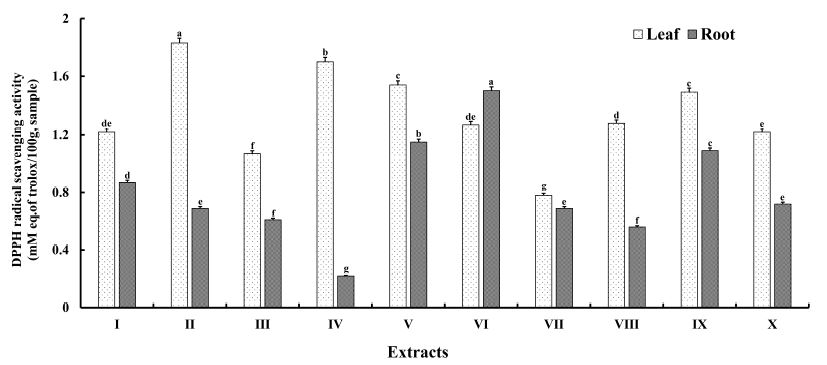
The DPPH radical scavenging activity of A. hookeri extracts obtained from different extracting solvents.1) Data represent the mean values for each sample ± standard deviation (n=5).2) a∼g Different superscripts within a column indicate the significant difference at the p<0.05 level.3) I=EtOAc, Ⅱ=EtOH, Ⅲ=DCM, IV=EtOAc 1/2 : EtOH 1/2, V=EtOH 1/2 : DCM 1/2, Ⅵ=DCM 1/2: EtOAc l/2; VII=EtOAc 1/3 : EtOH 1/3 : DCM 1/3, VIII=EtOAc 2/3 : EtOH 1/6 : DCM 1/6, IX=EtOAc 1/6 : EtOH 2/3 : DCM l/6; X=EtOAc 1/6 : EtOH 1/6 : DCM 2/3.
3. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay measures the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) in the presence of antioxidants, which acts as a reducing agent with a half-reaction reduction potential higher than that of the Fe3+/Fe2+ couple. This assay is used for the routine analysis of both single antioxidant and total antioxidative activities. The ferric-reducing abilities of A. hookeri extracts obtained using solvents of different polarities are presented in Fig. 3 and ranged from 22.85±0.87 to 249.45±9.53 mM TE for leaves and 3.67±0.14 to 75.80±2.90 mM TE for roots. The ferric-reducing ability of leaf extracts was in the order of II > I > VI > III > IV > X > VIII > IX > VII > V, while that of root extracts was in the order of IX > II > VIII > IV > V > VII > III > VI > X > I. In the case of leaf extracts, the ferric-reducing ability was highest (p<0.05) for extract II and lowest for extract V; similarly, the activity was highest for root extract II (p<0.05) and lowest for root extract I. The ferric-reducing abilities of the leaf extracts were higher than those of the root extracts except for the activities of extracts V, VII, and IX. Moreover, the values of ferric-reducing ability were consistently higher than those of the DPPH radical-scavenging activity. Thus, A. hookeri extracts were enriched with electron donors. Rafińska K et al (2019) reported that antioxidant capacity greatly relies on the solvent and plant part used for extraction. The polarity of the solvent used has an indirect effect on the extraction process because it may increase the solubility of antioxidant compounds (Alothman M et al 2009). Moreover, solvent type, polarity, and plant parts play important roles in determining the total reducing power (Do QD et al 2014; Ullah et al 2017). Different TPCs and TFCs and variations in antioxidant capacity may be attributed to the phytochemical PI and their association with the solvent PI. Solvents with similar PIs can dissolve phytochemicals that have similar or closely related PIs (Raman G et al 2005). Therefore, leaf extract II (PI=5.20) and root extract IX (PI=4.32) exhibited higher FRAP values than other leaf and root extracts. Phytochemicals could be extracted using solvents with a PI similar to their own, and their antioxidant capacities may vary accordingly.
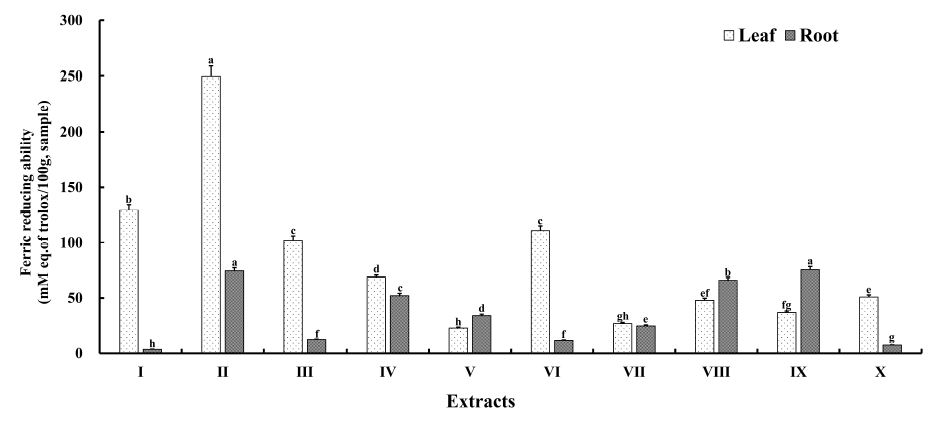
The ferric reducing ability of A. hookeri extracts obtained from different extracting solvents.1) Data represent the mean values for each sample ± standard deviation (n=5).2) a∼h Different superscripts within a column indicate the significant difference at the p<0.05 level.3) I=EtOAc, Ⅱ=EtOH, Ⅲ=DCM, IV=EtOAc 1/2 : EtOH 1/2, V=EtOH 1/2 : DCM 1/2, Ⅵ=DCM 1/2: EtOAc l/2; VII=EtOAc 1/3 : EtOH 1/3 : DCM 1/3, VIII=EtOAc 2/3 : EtOH 1/6 : DCM 1/6, IX=EtOAc 1/6 : EtOH 2/3 : DCM l/6; X=EtOAc 1/6 : EtOH 1/6 : DCM 2/3.
4. Total Antioxidant Capacity using the Trolox Equivalent Antioxidant Capacity (TEAC) Assay
The 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) assay is widely used to assess the antioxidant activity of vegetable and food matrices (Giurè AM et al 2017; Rameshwar Naidu J et al 2012; Shannon E et al 2018). This method is based on the quenching of stable colored radicals and serves as an indicator of the free-radical quenching activity of antioxidants even in complex biological matrices such as plants or food preparations (extracts or fractions). The total antioxidant activities of A. hookeri extracts obtained from different solvents were determined using the TEAC assay, and the results are presented in Fig. 4. The total antioxidant activity ranged from 2.48±0.07 to 9.15±0.26 mM TE for leaves and 0.64±0.02 to 7.01±0.19 mM TE for roots and was in the order of V > II > X > III > IV > VIII > IX > VII > VI > I for leaf extracts and V > II > IX > VIII > IV > X > VII > III > I > VI for root extracts. The total antioxidant activity was highest for leaf extract V (p<0.05) and lowest for extract I; similarly, the antioxidant activity of root extract V was highest (p<0.05) and that of root extract VI was lowest. The total antioxidant activities of leaf extracts were higher than those of root extracts. These findings are in agreement with previous results, wherein extracts obtained using solvent mixtures had stronger antioxidant capacities than those obtained using pure solvents (Do QD et al 2014). Thus, a solvent with lower polarity tends to be more effective in extracting radical-scavenging plant compounds than that with higher polarity. Bioactive compounds in leaves contain both simple and complex groups that differ in polarity. According to Zhou K & Yu L (2004), changes in solvent polarity alter the ability to dissolve a select group of antioxidant compounds and influence the antioxidant activity. Markom M et al (2007) found that a single solvent may or may not be selective for the separation of two components. Therefore, the extract V (PI=3.95) from the leaves and roots exhibited higher TEAC values than the other extracts. Antioxidant extraction may vary with the change in the solubility and polarity of antioxidants from plant materials in the extraction solvent.
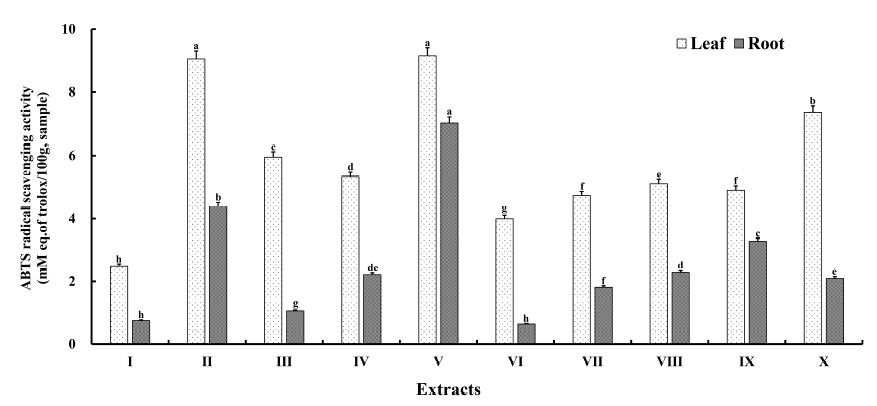
The ABTS radical scavenging activity of A. hookeri extracts obtained from different extracting solvents.1) Data represent the mean values for each sample ± standard deviation (n=5).2) a∼h Different superscripts within a column indicate the significant difference at the p<0.05 level.3) I=EtOAc, Ⅱ=EtOH, Ⅲ=DCM, IV=EtOAc 1/2 : EtOH 1/2, V=EtOH 1/2 : DCM 1/2, Ⅵ=DCM 1/2: EtOAc l/2; VII=EtOAc 1/3 : EtOH 1/3 : DCM 1/3, VIII=EtOAc 2/3 : EtOH 1/6 : DCM 1/6, IX=EtOAc 1/6 : EtOH 2/3 : DCM l/6; X=EtOAc 1/6 : EtOH 1/6 : DCM 2/3.
CONCLUSIONS
We investigated the effects of solvents with different polarities on the antioxidant activities of A. hookeri leaves and roots. In the case of leaves, extract VII (PI=3.89) and IV (PI=4.30) had the highest TPC and TFC, respectively. Extract II (PI=5.20) had the highest DPPH radical-scavenging activity and ferric-reducing ability. In the case of roots, extract II (PI=5.20) and III (PI=3.70) had the highest TPC and TFC, respectively, and extract VI (PI=3.55) and IX (PI=4.32) showed effective antioxidant activity (assessed using DPPH radical-scavenging activity) and ferric reducing ability, respectively. The total antioxidant activity was highest for extract V (PI=3.95) from both leaves and roots. As different phytochemicals were recovered using solvents with different polarities, the extraction solvent may play an important role when preparing A. hookeri extracts for use as nutraceuticals. Further studies are warranted to isolate and identify individual compounds from the crude extracts of A. hookeri based on the polarity of the extraction solvents.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
REFERENCES
-
Abdel-Aal EI, Haroon AM, Mofeed J (2015) Successive solvent extraction and GC-MS analysis for the evaluation of the phytochemical constituents of the filamentous green alga Spirogyra longata. Egypt J Aquat Res 41(3): 233-246.
[https://doi.org/10.1016/j.ejar.2015.06.001]

-
Abdullah N, Chin NL (2010) Simplex-centroid mixture formulation for optimised composting of kitchen waste. Bioresour Technol 101(21): 8205-8210.
[https://doi.org/10.1016/j.biortech.2010.05.068]

-
Alothman M, Bhat R, Karim AA (2009) UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov Food Sci Emerg Technol 10(4): 512-516.
[https://doi.org/10.1016/j.ifset.2009.03.004]

- Anwar F, Przybylski R (2012) Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Sci Pol Technol Aliment 11(3): 293-301.
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P (1994) Standardization of propolis extract and identification of principal constituents. J Pharm Belg 49(6): 462-468.
- Ayam VS (2011) Allium hookeri, Thw. Enum. A lesser known terrestrial perennial herb used as food and its ethnobotanical relevance in Manipur. Afr J Food Agric Nutr Dev 11(6): 5389-5412.
-
Baloch R, Uzair M, Chauhdary BA, Hayat MM, Alamgir M (2019) Phytochemical analysis, antioxidant and cytotoxic activities of Dryopteris ramosa. Biomed Res 30(5): 764-769.
[https://doi.org/10.35841/biomedicalresearch.30-19-321]

- Barchan A, Bakkali M, Arakrak A, Pagán R, Laglaoui A (2014) The effects of solvents polaritiy on the phenolic contents and antioxidant activity of three Mentha species extracts. Int J Curr Microbiol App Sci 3(11): 399-412.
-
Barwick V (1997) Strategies for solvent selection–a literature review. Trends Anal Chem 16(6): 293-309.
[https://doi.org/10.1016/S0165-9936(97)00039-3]

-
Bimakr M, Rahman RA, Taip FS, Ganjloo A, Salleh LM, Selamat J, Hamid A, Zaidul ISM (2011) Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod Process 89(1): 67-72.
[https://doi.org/10.1016/j.fbp.2010.03.002]

-
Block E, Naganathan S, Putman D, Zhao SH (1992) Allium Chemistry : HPLC analysis of thiosulfinates from onion, garlic, wild garlic (ramsoms), leek, scallion, shallot, elephant (great-headed) garlic, chive, and Chinese chive. Uniquely high allyl to methyl ratios in some garlic samples. J Agric Food Chem 40(12): 2410-2430.
[https://doi.org/10.1021/jf00024a017]

-
Cos P, Vlietinck AJ, Berghe DV, Maes L (2006) Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J Ethnopharmacol 106(3): 290-302.
[https://doi.org/10.1016/j.jep.2006.04.003]

-
Cowan MM (1999) Plant product as antimicrobial agents. Clin Microbiol Rev 12(4): 564-582.
[https://doi.org/10.1128/CMR.12.4.564]

- Das K, Tiwari RKS, Shrivastava DK (2010) Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J Med Plants Res 4(2): 104-111.
-
Dehkharghanian M, Adenier H, Vijayalakshmi MA (2010) Analytical methods study of flavonoids in aqueous spinach extract using positive electrospray ionisation tandem quadrupole mass spectrometry. Food Chem 121(3): 863-870.
[https://doi.org/10.1016/j.foodchem.2010.01.007]

-
Delgado-Andrade C, Rufián-Henares JA, Morales FJ (2005) Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J Agric Food Chem 53(20): 7832-7836.
[https://doi.org/10.1021/jf0512353]

-
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22(3): 296-302.
[https://doi.org/10.1016/j.jfda.2013.11.001]

-
Fernández-Agulló A, Pereira E, Freire MS, Valentão P, Andrade PB, González-Álvarez J, Pereira JA (2013) Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod 42: 126-132.
[https://doi.org/10.1016/j.indcrop.2012.05.021]

-
Ghasemzadeh A, Jaafar HZ, Juraimi AS, Tayebi-Meigooni A (2015) Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of hashemi rice bran. Molecules 20(6): 10822-10838.
[https://doi.org/10.3390/molecules200610822]

- Ghasemzadeh A, Jaafar HZ, Rahmat A (2011) Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe) extracts. J Med Plants Res 5(7): 1147-1154.
- Giurè AM, Zappia C, Capocasale M (2017) Physico-chemical stability of blood orange juice during frozen storage. Int J Food Prop 20(2): 1930-1943.
-
Houghton PJ, Raman A (1998) Laboratory Handbook for the Fractionation of Natural Extracts. Chapman and Hall, New York. p 199.
[https://doi.org/10.1007/978-1-4615-5809-5]

-
Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL, Haines DD (2015) Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes 8(1): 396.
[https://doi.org/10.1186/s13104-015-1388-1]

-
Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG, Scherwitz L, Beaman R, Endres JR, Schauss AG (2008) In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem 56(18): 8326-8333.
[https://doi.org/10.1021/jf8016157]

-
Lapornik B, Prošek M, Wondra AG (2005) Comparison of extracts prepared from plant by-products using different solvents and extraction time. J Food Eng 71(2): 214-222.
[https://doi.org/10.1016/j.jfoodeng.2004.10.036]

-
Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC (2010) Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem 21(8): 717-725.
[https://doi.org/10.1016/j.jnutbio.2009.04.012]

-
Lee HJ, Baik JE, Joo NM (2014) Quality characteristics and storage stability of bread with Allium hookeri powder. J Korean Soc Food Sci Nutr 27(2): 318-329.
[https://doi.org/10.9799/ksfan.2014.27.2.318]

-
Lee KW, Kim YS, Park PJ, Jeong JH (2014) Comparison of effect of water and ethanolic extract from roots and leaves of Allium hookeri. J Korean Soc Food Sci Nutr 43(12): 1808-1816.
[https://doi.org/10.3746/jkfn.2014.43.12.1808]

-
Markom M, Hasan M, Daud WRW, Singh H, Jahim JM (2007) Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep Purif Technol 52(3): 487-496.
[https://doi.org/10.1016/j.seppur.2006.06.003]

-
Moon JK, Shibamoto T (2009) Antioxidant assays for plant and food components. J Agric Food Chem 57(5): 1655-1666.
[https://doi.org/10.1021/jf803537k]

-
Nyiredy S (2004) Separation strategies of plant constituents–current status. J Chromatogr B 812(1-2): 35-51.
[https://doi.org/10.1016/S1570-0232(04)00719-6]

-
Quezada N, Cherian G (2012) Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur J Lipid Sci Technol 114(8): 974-982.
[https://doi.org/10.1002/ejlt.201100298]

-
Rafińska K, Pomastowski P, Rudnicka J, Krakowska A, Maruśka A, Narkute M, Buszewski B (2019) Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem 289(15): 16-25.
[https://doi.org/10.1016/j.foodchem.2019.03.025]

-
Raman G, Cho M, Brodbelt JS, Patil BS (2005) Isolation and purification of closely related citrus limonoid glucosides by flash chromatography. Phytochem Anal 16(3): 155-160.
[https://doi.org/10.1002/pca.835]

- Rameshwar Naidu J, Ismail RB, Chen Y, Sasidharan S, Kumar P (2012) Chemical composition and antioxidant activity of the crude methanolic extracts of Mentha spicata. J Phytol 4(1): 13-18.
-
Rasera GB, Hilkner MH, de Alencar SM, de Castro RJS (2019) Biologically active compounds from white and black mustard grains: An optimization study for recovery and identification of phenolic antioxidants. Ind Crops Prod 135: 294-300.
[https://doi.org/10.1016/j.indcrop.2019.04.059]

-
Rhyu DY, Park SH (2013) Characterization of alkyl thiosulfinate in Allium hookeri root using HPLC-ESI-MS. J Korean Soc Appl Bi 56(4): 457-459.
[https://doi.org/10.1007/s13765-013-3069-x]

-
Rose P, Whiteman M, Moore PK, Zhu YZ (2006) Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat Prod Rep 22(3): 351-368.
[https://doi.org/10.1039/b417639c]

-
Shannon E, Jaiswal AK, Abu-Ghannam N (2018) Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Res 2(1): 1-11.
[https://doi.org/10.26656/fr.2017.2(1).117]

-
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol 299:152-178.
[https://doi.org/10.1016/S0076-6879(99)99017-1]

-
Snyder LR, Dolan JW, Carr PW (2004) The hydrophobic-subtraction model of reversed-phase column selectivity. J Chromatogr A 1060(1-2): 77-116.
[https://doi.org/10.1016/S0021-9673(04)01480-3]

-
Soares PK, Bruns RE, Scarminio IS (2009) Statistical mixture design investigation of fractionated and total extracts from Erythrina speciosa Andrews leaves. J Sep Sci 32(4): 644-652.
[https://doi.org/10.1002/jssc.200800534]

-
Thoo YY, Ho SK, Liang JY, Ho CW, Tan CP (2010) Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from Mengkudu (Morinda citrifolia). Food Chem 120(1): 290-295.
[https://doi.org/10.1016/j.foodchem.2009.09.064]

- Ullah I, Wakeel A, Shinwari ZK, Jan SA, Khalil AT, Ali M (2017) Antibacterial and antifungal activity of Isatis tinctoria L. (Brassicaceae) using the micro-plate method. Pak J Bot 49(5):1949-1957.
-
Valente LMM, da Paixão D, do Nascimento AC, dos Santos PFP, Scheinvar LA, Moura MRL, Tinoco LW, Gomes LNF, da Silva JFM (2010) Antiradical activity, nutritional potential and flavonoids of the cladodes of Opuntia monacantha (Cactaceae). Food Chem 123(4): 1127-1131.
[https://doi.org/10.1016/j.foodchem.2010.05.074]

-
Waszkowiak K, Gliszczyńska-Świgło A, Barthet V, Skręty J (2015) Effect of extraction method on the phenolic and cyanogenic glucoside profile of flaxseed extracts and their antioxidant capacity. J Am Oil Chem Soc 92(11-12): 1609-1619.
[https://doi.org/10.1007/s11746-015-2729-x]

-
You BR, Kim EG, Choi HJ, Kim HJ (2013) Quality characteristics of kimchi with Allium hookeri root powder added. Korean J Food Preserv 20(6): 863-870.
[https://doi.org/10.11002/kjfp.2013.20.6.863]

-
You S, Jang M, Kim G (2018) Antioxidant activity and neuroprotective effect of root bark of Morus alba L. extract against hydrogen peroxide-induced cytotoxicity in PC12 cells. J Korean Soc Food Sci Nutr 47(5): 519-527.
[https://doi.org/10.3746/jjkfn.2018.47.5.519]

-
Yu Lin H, Kuo YH, Lin YL, Chiang W (2009) Antioxidative effect and active component from leaves of lotus (Nelumbo nucifera). J Agric Food Chem 57(15): 6623-6629.
[https://doi.org/10.1021/jf900950z]

-
Zhou K, Yu L (2004) Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT - Food Sci Technol 37(7): 717-721.
[https://doi.org/10.1016/j.lwt.2004.02.008]


