
The Impact of Very Low-Calorie Diet-Induced Weight Loss on Changes in the Serum Vitamin D Levels, Insulin Resistance, and Inflammatory Biomarkers in Overweight Korean Women
Abstract
Obese patients often have low serum levels of 25-OH-vitamin D (25-OH-D), and this may induce insulin resistance and cardiovascular disease. In this study, we investigated whether a reduction in body weight, as achieved within a short period by putting patients on a very low-calorie diet (VLCD), improved the serum 25-OH-D levels. Then we performed a secondary analysis of data collected from a previous study on the impact of a lemon detox program on body fat reduction. We further analyzed any potential correlation between alterations in 25-OH-D levels and changes in biomarkers of insulin resistance and inflammation, which are both related to cardiovascular disease, after weight reduction. A total of 84 pre-menopausal overweight women, who consumed either a VLCD (about 400 kcal/day) or a normal diet for 11 days, were divided into 4 groups by quartiles based on their degree of weight loss. The average serum 25-OH-D level of the entire cohort before the trial was 11.7±3.9 ng/mL, and this level significantly declined after the intervention. However, in the group with the highest weight loss (Q1), which experienced an average body weight reduction of 5.7%, the serum 25-OH-D levels were slightly increased following the weight loss. Further, this increase was significantly greater than that of the other weight loss groups. Weight-loss-induced elevation of serum 25-OH-D levels was significantly associated with a reduction in body weight (p<0.01), body mass index (p<0.05), waist-hip ratio (p<0.05), waist circumference (p<0.01), total body fat mass (p<0.05), serum adiponectin (p<0.01), insulin-like growth factor-1 (p<0.05), and triglycerides (p<0.05). Additionally, as compared to the group with the lowest weight loss, the group with the highest weight loss experienced the largest declines in serum glucose, glycosylated hemoglobin (HbA1c), insulin, and leptin levels and finally insulin resistance. All this data suggests that a loss of 5% or more of body weight can improve the serum 25-OH-D levels and the factors contributing to diabetes and cardiovascular disease.
Keywords:
25-OH-vitamin D, very low-calorie diet, weight loss, insulin resistance, cardiovascular diseaseINTRODUCTION
Obesity is a risk factor for chronic diseases such as hypertension, diabetes, cardiovascular disease, and cancer, which are gradually increasing (Broussard JL et al 2016). According to Korean statistics, the adult obesity rate in Korea has rapidly increased from 24.3% in 1998 to 34.8% in 2016, with 42.3% of men and 26.4% of women considered obese (Statistics Korea 2018). This trend correlates with an increased consumption of dietary fat and simple sugars.
Vitamin D is a pro-hormone that promotes calcium absorption (Hatfield DP et al 2014); it is therefore linked to diseases associated with calcium metabolism. Inadequate vitamin D is a risk factor for skeletal, muscular, and cardiovascular diseases, as well as cancer, multiple sclerosis, and diabetes (Grant WB & Holick MF 2005). There has been much interest in the non-skeletal effects of vitamin D deficiency in recent years, particularly the effects related to cardiovascular disease, diabetes mellitus, cancer, and immune dysfunction (Gannagé-Yared MH et al 2009; Anderson JL et al 2010; Rosen CJ 2011). It has been observed that an increase in serum 25-hydroxyvitamin D (25-OH-D) concentrations cause a significant decrease in fasting glucose and insulin resistance, and vitamin D deficiency is a known risk factor for type 2 diabetes and the metabolic syndrome (Gannagé-Yared MH et al 2009). It has also been reported that a rapid reduction of the body weight by 4% significantly decreases leptin and insulin levels, and activates beta-cell function (Mohammad T et al 2015). In addition, vitamin D supplementation plays an important role in the management of cardiovascular disease (Brandenburg VM et al 2012). Therefore, vitamin D has been proposed to be a preventive factor in chronic diseases such as obesity, diabetes, and cardiovascular disease.
The serum 25-OH-D level reflects the total body vitamin D status since both endogenous synthesis after ultraviolet (UV) light exposure and dietary intake from food sources are incorporated in this index. The Endocrine Society determined that serum 25-OH-D levels of 30 ng/mL or above are normal, and levels below 20 ng/mL indicate deficiency; these values are used as the standard for assessment of the nutritional vitamin D status in Korea (Lim S et al 2012). The prevalence of inadequate vitamin D levels in Korea is high and is increasing over time due to altered life-style patterns, which include more time spent indoors. In their study, Choi HS et al (2011) found that the vitamin D deficiency rates in Korean men and women were 47.3% and 64.5%, respectively, with the highest proportion observed in women in their 20s (79.9%). According to a study by Park JH et al (2018), vitamin D deficiency is increasing; the reported rates were 75.2% and 82.5% in men and women, respectively, in 2014 compared to corresponding values of 51.8% and 68.2%, respectively, in 2008. The causes of very low 25-OH-D serum levels (below 10 ng/mL) include poor dietary intake of vitamin D coupled with negligible sun exposure, malabsorption due to inflammatory bowel disease, gluten enteropathy, gastric surgery, biliary disease, intestinal bacterial overgrowth, use of antiseizure medications, and long-term use of glucocorticoids (DeLuca HF 2004).
Several epidemiological and experimental studies have reported a link between obesity and low serum 25-OH-D levels. A study by Blum M et al (2008) suggests that serum 25-OH-D levels are inversely correlated with body mass index (BMI); moreover, an elevation in the BMI by 5 kg/m2 or a gain in body weight by 15 kg resulted in a decrease in 25-OH-D concentrations by 4 ng/mL in healthy older participants. Several clinical trials also indicate that obesity might lead to a lack of outdoor activities and a consequent decrease in vitamin D synthesis in the skin (Wortsman J et al 2000; Willis CM et al 2007); it also leads to reduced 25-OH-D release from the adipose tissue owing to vitamin D accumulation in the fat cells (Shantavasinkul PC et al 2015). Since body fat mass is increased in obesity and vitamin D is fat soluble and readily stored in adipose tissue, it may be presumed that obese individuals have large stores of vitamin D (Wortsman J et al 2000). However, the Korean Nutrition Society recommends higher vitamin D intakes for obese people because the vitamin D accumulated in fat tissue may be inaccessible (Korea Ministry of Health & Welfare 2015). In view of these observations, this study intended to evaluate whether serum 25-OH-D levels may be restored by weight loss in obese individuals.
Studies on vitamin D in Korea have focused on the BMI or metabolic syndrome in post-menopausal women or middle-aged adults (Jang SY et al 2012; Kim YJ et al 2012). Potential improvements in serum 25-OH-D levels by weight loss, or the mechanisms underlying low serum vitamin D levels in obese pre-menopausal women, have not been assessed. The very low-calorie diet (VLCD) is a weight loss program that limits calorie intake to less than 500 kcal/day. This diet can reduce body weight rapidly within a short period of time, but may also cause imbalances in the vitamin and mineral reserves of the body (Henry RR et al 1986), causing symptoms such as headache, dizziness, fatigue, and muscular aches (Delbridge E & Proietto J 2006; Baker S et al 2009). The lemon detox program is a type of VLCD for reducing body weight and fat and is designed to provide higher amounts of vitamin C and minerals than other VLCDs (Beyer KA 2006). We hypothesized that body weight reduction induced by VLCDs, including the lemon detox program, would restore serum 25-OH-D levels and improve cardiovascular and diabetic risk factors related to 25-OH-D without causing imbalances in vitamins and minerals in obese individuals. To verify our hypothesis, we performed a secondary analysis of data collected in our previous study, in which healthy pre-menopausal women were subjected to a short-term weight loss intervention with the lemon detox program (Kim MJ et al 2015). Participants were divided into 4 groups according to their weight loss; the changes in serum 25-OH-D levels, body composition, homeostasis model assessment insulin resistance (HOMA-IR) scores, blood insulin resistance, adipokines, and levels of inflammatory biomarkers associated with cardiovascular risk factors were also compared.
STUDY METHODS
1. Participants and Study Design
As this study was a secondary analysis of our previous study on the weight loss impact of the lemon detox program (Kim MJ et al 2015), we used the same data set from that study, which was approved by the Institutional Review Board of the Seoul Women's University (IRB-2013A-4). The participants included 84 healthy pre-menopausal women aged between 20 and 50 years, with a BMI ≥23 kg/m2, who did not have diabetes or any other major medical conditions, and were consuming a regular Korean diet. The clinical intervention period lasted 11 days; the recruitment of participants and our experimental intervention trial, including the nutrient content for each ingredient, and the dietary intakes of the lemon detox juice- and placebo juice-consuming groups, have been reported previously (Kim MJ et al 2015). The VLCD group comprised participants consuming either lemon detox (409.3 kcal per a day) or placebo (403.2 kcal per a day) juice; they were divided into 4 groups according to quartiles based on their final weight loss. The body weight of participants in the group with the highest weight loss decreased by more than 3.2 kg (Q1 group). In the group with the second (Q2), third (Q3), and fourth (Q4) highest weight loss, participants lost between ≥1.7 and <3.2 kg, ≥0.7 and <1.7 kg, and <0.7 kg of body weight, respectively.
2. General Characteristics and Dietary Intakes
Data on general participant characteristics (age, occupation, household income, educational status, and marital status) were collected by a self-administered questionnaire survey. In order to identify differences in the household income between the weight loss groups, we divided the participants into 2 income categories of less than 5,000,000 won and more than 5,000,000 won. Factors related to obesity and weight loss, such as experience with previous weight loss programs, number of previous participations, and perception of current weight, were assessed. Data on vitamin D intake were obtained to investigate the differences in vitamin D status between the weight loss groups.
3. Anthropometric and Biochemical Parameters
To determine the impact of weight loss, anthropometric parameters, including height and weight, lean body mass, body fat mass, body fat percentage, BMI, waist-hip ratio (WHR), bone mineral density (BMD, compared with healthy adults of the same age by t-score), and the BMD of vertebrae 1∼4 of the lumbar spine, were assessed before and after the trial. The extent of weight loss was calculated from the difference between body weight before and after the intervention.
To determine whether the vitamin D status recovered after losing body weight, the serum 25-OH-D levels were evaluated by a chemiluminescence immunoassay using a commercial kit (Liaison Total 25-OH-Vitamin D kit, Diasorin, Stillwater, Minnesota, USA). Serum lipid profiles, including serum triglycerides (TG), total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and free fatty acid levels were assessed. To investigate the impact of weight loss on factors related to insulin resistance, the levels of fasting serum glucose, HbA1c, serum insulin levels, and the HOMA-IR score (according to Matthews DR et al (1985)) were examined. Serum leptin and adiponectin levels were also included in the analysis as these adipokines are associated with insulin resistance and cardiovascular diseases. We also investigated the levels of serum insulin-like growth factor-1 (IGF-1), homocysteine, and highsensitivity C-reactive protein (hs-CRP) as inflammatory biomarkers associated with the early inflammatory events of cardiovascular disease (Ridker PM et al 2000). The tests for all the serum biochemical indices, except serum 25-OH-D, have been described in our previous study (Kim MJ et al 2015).
4. Statistical Analysis
All statistical analyses for this study were performed using the SPSS program (IBM SPSS Statistics version 21, SPSS Inc, Chicago, Illinois, USA). All data have been presented as means±standard deviation (SD). Clinical data obtained before and after the intervention were compared using a paired t-test. One-way analysis of variance (ANOVA) and Duncan’s multiple range tests were performed to identify significant differences between the quartile groups in terms of the degrees of loss in body weight. To exclude the effect of dietary vitamin D intake, analysis of covariance was performed using a general linear model to compare the changes in serum 25-OH-D levels among the weight-loss groups. General characteristics have been presented as both numbers and percentages, and significant differences between the quartile groups were determined by χ2-tests. A p<0.05 was considered statistically significant. Correlations between serum 25-OH-D and other variables were established using the Pearson’s and partial correlation tests, adjusted for changes in vitamin D intake.
RESULTS AND DISCUSSION
1. Participant Demographics and Weight Loss
The average age of the cohort was 24.2±7.5 (Q1: 23.0±5.6, Q2: 25.1±8.1, Q3: 25.4±9.3, Q4: 23.5±7.0) years. Most of the participants were students (72.6%), were unmarried (86.9%), and had an education of higher than the college level (88.1%) (data not shown). General characteristics of the participants including occupation, educational level, family income, and marital status did not significantly differ between the quartile groups, categorized by extent of weight loss. Overall, 76 women stated that they had attempted weight loss before; 51.3% had attempted it more than thrice. However, there were no significant differences between the weight loss quartile groups. All participants considered themselves to be overweight (50.0%) or obese (50.0%); the proportion of subjects who recognized themselves as obese (76.2%) was significantly higher in the group with the highest weight loss (Q1) than in the other quartile groups (Table 1).
Schaumberg K et al (2015) demonstrated that overweight young women lost significantly more weight than those with a healthy weight at the beginning of a weight loss program, and suggested that women who perceive themselves as overweight are more motivated to lose weight. In their study, Czeglédi E (2017) reported that the acceptable weight loss percentage was higher in younger participants with excess weight, and the motivation for weight loss was linked to dissatisfaction with their body image. In this study, the highest weight loss was achieved by women in the Q1 group. Since a higher proportion of these women recognized themselves as obese, this probably reflected a greater desire or motivation for weight loss in the Q1 group than in the other groups.
2. Anthropometric Parameters and Bone Mineral Density
The average height, body weight, and BMI of all subjects before the intervention were 162.2 cm, 68.3 kg, and 25.9 kg/m2, respectively. The average weight and BMI were both significantly reduced by the intervention (weight: 66.4 kg, BMI: 25.2 kg/m2). Likewise, a significant reduction in waist circumference, WHR, trunk and total fat mass, and body fat percentage was achieved by the intervention. Comparing the 4 quartile groups, the declines in the BMI, WHR, and trunk and total fat mass were significantly higher in the Q1 group than in the Q3 and Q4 groups. However, no significant differences were observed between the groups in terms of changes in waist circumference and percentage of body fat (Table 2). The average weight loss of participants in the 2 dietary restriction groups (lemon detox and placebo) was lower than that of the regular diet group (data not shown). In terms of quartile groups, 72.2% of the participants on the VLCD were in the Q1 (average weight loss 5.7%) and Q2 (average weight loss 3.8%) groups, and dietary restriction significantly affected weight loss. Several studies have reported reductions in body weight, BMI, abdominal fat, and visceral fat with diets that restricted energy intake to less than 1,000 kcal/day for 9 to 20 weeks (Janssen I et al 2002; Laaksonen DE et al 2003). Although the duration of this study was considerably short compared to that of other studies, we still observed remarkable declines in body weight, BMI, body fat percentage, WHR, and waist circumference owing to the dietary restriction.
Except for Q4 group, the average t-score was significantly increased by the intervention in all weight loss groups. In particular, the weight-loss-induced increases in the BMD of vertebrae 1∼4 of the lumbar spine were significantly higher in Q1, Q2, and Q3 groups than in Q4 group (Table 2). Gossain VV et al (1999) evaluated the impact of weight loss on the BMD in obese women; after consuming an 800-calorie diet for 12 weeks, the cohort experienced a noticeable decline in total BMD without remarkable changes in the mineral density of the lumbar spine. Nishizawa Y et al (1992) suggested that VLCD may cause bone mineral changes that may disturb calcium homeostasis. However, these effects appeared to vary between different bones of the body; the mineral content of the cranial bones increased, while that of bones in the legs decreased. In contrast, in our study, we observed a significant increase in the lumbar spine BMD and no changes in total BMD after dietary restriction. These results agree with those of previous studies, in which the impact of VLCD on BMD varied by body region. In addition, the BMD did not significantly correlate with the masses of fat or lean body (data not shown). Since our program lasted for only 11 days and did not induce a weight reduction comparable to long-term weight loss programs, it did not apparently cause any changes in BMD.
3. Serum 25-OH-D Levels and Dietary Vitamin D Intake
In our study, the average serum 25-OH-D level of the entire cohort was 11.67 ng/mL before the intervention; this level was indicative of a vitamin D-deficient state. Dietary restriction caused a further reduction of this level to 10.95 ng/mL. Decreased dietary vitamin D intake was observed in all groups after the intervention; this may have been caused by calorie restriction in the VLCDs. However, the absolute changes were not significantly different between the groups (Table 3). Since it has a long half-life and is not tightly regulated to maintain blood homeostasis, serum 25-OH-D is a good indicator of vitamin D status, and reflects both, oral intake and skin synthesis of vitamin D (Hatfield DP et al 2014). Compared to healthy people, patients with obesity have been observed to have lower serum vitamin D levels, and about 60% of seriously obese people are considered vitamin D-deficient (Carlin AM et al 2006; Goldner WS et al 2008).
However, on comparing the changes in serum 25-OH-D levels based on weight reduction among the quartile weight loss groups, serum 25-OH-D levels were significantly decreased in groups with less than 5% weight loss after the intervention (Q2, Q3, and Q4 groups). Conversely, the Q1 group with an average weight loss of 5.7% showed a slight increase in serum 25-OH-D levels. However, the difference was not significant. Notably, serum 25-OH-D levels were significantly decreased in the Q2 group, which experienced the least reduction in vitamin D intake. Although the vitamin D intake in Q1 group was lower than that in Q2 group, the serum levels rose slightly in this group (Table 3).
It is possible that the reduced serum 25-OH-D concentration in the group that lost less than 5% of body weight after the intervention was probably related to the decrease in vitamin D intake on the VLCD. Therefore, to remove the confounding influence of reduced vitamin D intake during calorie restriction on changes in serum 25-OH-D levels, we analyzed the changes in serum 25-OH-D levels between the weight loss groups after statistical adjustment for changes in vitamin D intake. The increase in the serum 25-OH-D level was significantly higher in the group with the highest weight loss than in groups with other weight loss; the decline in the serum 25-OH-D level in the Q2 group, which lost more weight than the Q3 group, was significantly lower than that in the Q3 group (Fig. 1). Since a certain level of weight loss affected the serum 25-OH-D level, these results suggest that the serum 25-OH-D level may be elevated in groups experiencing considerable weight loss, despite decreased dietary vitamin D intakes. Since vitamin D is fat soluble, the reduction of body fat mass in obese patients might reduce the stores of vitamin D, which leads to increased levels in the blood (Wortsman J et al 2000). Several clinical trials have indicated that serum 25-OH-D levels decrease with weight gain and recover with weight loss (Parikh SJ et al 2004; Tzotzas T et al 2010).
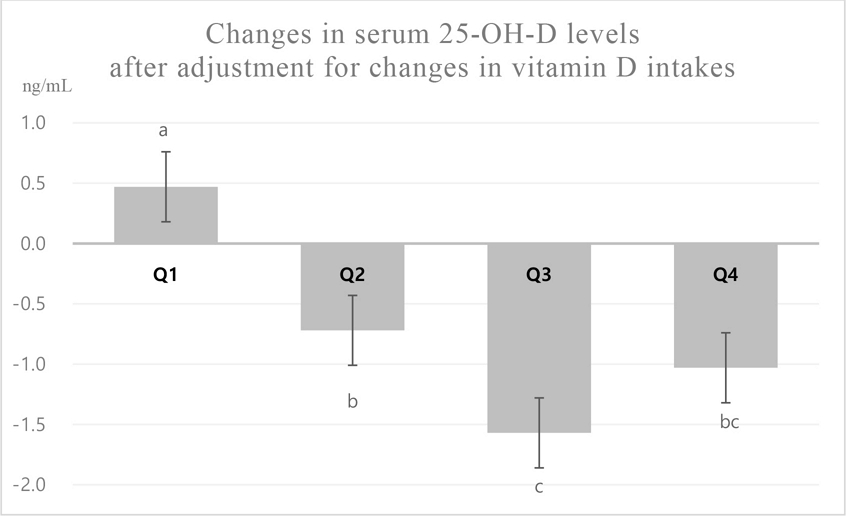
Changes in serum 25-OH-D levels after adjustment for changes in vitamin D intakes across the weight loss groups.Values are estimated means±Standard error; estimated means were determined using a general linear model after adjustment for changes in vitamin D intake.a∼c Significantly differed (at p<0.05) between the quartile weight loss groups on analysis of covariance and least significant difference multiple range tests.Q1, weight loss ≥ 3.2 kg (mean weight loss 5.7%); Q2, 1.7 kg ≤ weight loss < 3.2 kg (mean weight loss 3.8%); Q3, 0.7 kg ≤ weight loss < 1.7 kg (mean weight loss 1.8%); Q4, weight loss < 0.7 kg (mean weight loss 0.3%).
However, we observed an increased serum 25-OH-D level after weight reduction only in the Q1 group; this increase was not significantly different from the level prior to weight reduction. In a study by Rock CL et al (2012), serum 25-OH-D levels increased when the body weight reduced by 5% or more following a 24-month long-term weight loss program. Furthermore, a study employing a diet-restricted exercise regimen demonstrated elevated serum 25-OH-D concentrations with body weight reductions of 5% or more (Mason C et al 2011). The results of our study corroborate these observations, in that serum 25-OH-D levels increased when the body weight was reduced by an average of 5.7%. We speculated that the increase in serum 25-OH-D induced by weight loss in the Q1 group was not significant because the VLCD intervention extended for only 11 days; this may not have been adequate in restoring blood vitamin D levels by releasing accumulated vitamin D from body fat. Therefore, we speculate that the weight reduction in the Q1 group may represent a cut-off point for the recovery of serum 25-OH-D levels, and further weight loss over prolonged periods may potentially elevate these levels.
4. Serum Glucose, HbA1c, Insulin, and Adipokine Levels and Insulin Resistance
Serum glucose, insulin levels, and HbA1c content were significantly decreased by the intervention in the Q1 and Q2 groups, and these declines were significantly greater in the Q1 group than in the Q4 group. However, it did not differ between the Q2 group and Q3 and Q4 groups. The HOMA-IR score, which indicates insulin resistance, was significantly reduced in Q1 and Q2 groups, and the decrease was significantly greater in both groups than in the Q4 group. The levels of fasting plasma glucose, HbA1c, serum insulin, and the HOMA-IR score were significantly reduced in the group with the highest weight loss, indicating a significant improvement in blood glucose-related metabolism (Table 4).

Changes in the levels of serum glucose, HbA1c, insulin, and adipokines and in HOMA-IR scores after the intervention
Tzotzas T et al (2010) reported that insulin resistance, including serum insulin levels and the HOMA-IR score, improved after a 10% reduction in body weight following 20 weeks on a low-calorie diet. Calorie restriction is known to improve the levels of adipokines such as leptin and adiponectin, that are produced in adipocytes (Laaksonen DE et al 2003). Leptin is an indicator of obesity and cardiovascular disease, and may induce insulin resistance (Havel PJ 2002). In our study, serum leptin levels decreased in the Q1 and Q2 groups, and the reduced leptin concentrations correlated with the decrease in body weight. Therefore, despite the short study period, dietary restriction using a VLCD seemed to exert a positive effect on leptin levels and insulin resistance. Serum adiponectin levels are typically low in obese patients (Kardas F et al 2013); in this study, they significantly decreased in Q1, Q2, and Q3 groups, but not in Q4 group (Table 4).
5. Serum Lipid Profiles and Cardiovascular Disease-related Inflammatory Biomarkers
Serum lipid levels (total cholesterol, TG, HDL-C, and LDL-C) typically decrease after dietary restriction. We observed a significant decrease in total cholesterol (p<0.001), TG (p<0.01), HDL-C (p<0.001), and LDL-C (p<0.01) levels in the Q1 group. Free fatty acid concentrations increased in all 4 groups, with the highest increase detected in the Q1 group (p<0.001, Table 5).
Elevated serum TG, total cholesterol, and LDL-C levels are risk factors for cardiovascular disease. Therefore, reductions in these lipids after dietary restriction are beneficial for the cardiovascular disease index. In contrast, although HDL-C levels were reported to increase in obese patients following a calorie restricted diet, they decreased in our study. This may be attributed to the extremely limited amount of food and nutrients in our experimental diet. Krikken JA et al (2012) reported that short-term sodium restriction decreased HDL-C levels by affecting the glomerular filtration of HDL apolipoproteins, thereby stimulating HDL catabolism.
In states of fasting or short-term starvation, levels of serum free fatty acids rise owing to the release of fatty acids from adipose tissue induced by increases in glucagon (Joesting JJ et al 2014). In our study, the free fatty acid concentration was found to be significantly increased by the intervention, particularly in the Q1 group. Since this group experienced the highest weight loss, the observed changes in free fatty acids may be attributed to the fact that participants in the Q1 group followed the dietary restrictions more carefully (Huffman KM et al 2012).
The serum levels of leptin and IGF-1 significantly decreased in the Q1 (p<0.001) and Q2 (p<0.001) groups after dietary restriction (Table 5). Studies that administered restricted diets to rodents with cancer showed that IGF-1 levels were lowered by the dietary intervention, thereby increasing apoptosis, decreasing cell proliferation, and consequentially depressing tumor progression (Hursting SD et al 1993; Harvey AE et al 2013). McCarty MF (1999) suggested that reduced IGF-1 levels caused by increased glucagon levels could inhibit the hyperproliferation of endometrial smooth muscle, thereby lowering the risk of cardiovascular disease.
The levels of serum adiponectin, which is known to protect from cardiovascular disease (Zoccali C et al 2002), significantly decreased in all groups except for Q4 group, with a significantly greater decline observed in Q1 group than in other groups (p<0.001, Table 4). Other long-term clinical trials that extended for 5 to 6 months observed increases in the plasma adiponectin concentration following weight loss (Golubović MV et al 2013; Harvey AE et al 2013); our study period was probably inadequate for observing improvements in adiponectin levels.
The serum homocysteine levels increased after dietary restriction in groups Q1 and Q2. Serum hs-CRP levels did not significantly change after weight loss in any group (Table 5). It is known that oral vitamin intake can inhibit increases in homocysteine levels during weight loss (Henning BF et al 1998). Nevertheless, we attribute the increase in homocysteine levels observed in our study to an insufficient intake of folic acid, which is required for homocysteine catabolism (Beck B et al 2012). In addition, in the participants of our study, a trend towards lower hs-CRP levels was observed despite the short study period; this suggests that in cardiovascular diseases such as myocardial infarction, the VLCD may exert certain inhibitory effects on the inflammatory response.
6. Correlation between Changes in Serum 25-OH-D Levels with Changes in Anthropometric and Biochemical Parameters
The changes in serum 25-OH-D levels were significantly negatively correlated with changes in body weight (r=—0.369, p<0.01), BMI (r=—0.335, p<0.05), WHR (r=—0.277, p<0.05), waist circumference (r=—0.333, p<0.05), and total body fat mass (r=—0.260, p<0.05). In terms of biochemical parameters, changes in serum 25-OH-D levels correlated negatively with changes in IGF-1 (r=—0.241, p<0.05), adiponectin (r=—0.283, p<0.01), TG (r=—0.279, p<0.05), and HDL-C (r=—0.238, p<0.05) after the VLCD intervention, but correlated positively with changes in homocysteine (r=0.220, p<0.05) and uric acid (r=0.322, p<0.01) levels (Table 6).

Correlation between changes in serum 25-OH-D levels and changes in anthropometric and biochemical parameters after the intervention
Several clinical trials have reported a negative correlation between serum 25-OH-D levels and body weight, BMI, and body fat mass; serum 25-OH-D was found to be noticeably lower in obese participants than in their healthy counterparts (Parikh SJ et al 2004; Tzotzas T et al 2010). Despite the short study period, we also observed a negative correlation between the serum 25-OH-D level and anthropometric measurements such as body weight, BMI, WHR, waist circumference, and total body fat mass. These results were similar on partial correlation, statistically adjusted for changes in vitamin D intake (body weight (r=—0.377, p<0.001), BMI (r=—0.343, p<0.01), WHR (r=—0.280, p<0.05), waist circumference (r=—0.331, p<0.05), and total body fat mass (r=—0.267, p<0.05). In a study that administered 700 IU of vitamin D daily to older adults aged over 65 years for 1 year, the serum 25-OH-D concentration decreased by approximately 4 ng/mL (10 nmol/L) for every 15 kg of increased body weight compared to the baseline, and showed an inverse correlation with the BMI (Blum M et al 2008). Wortsman J et al (2000) had observed that in obese patients, the vitamin D accumulated in adipose tissue could not be released into the blood as required. They suggested that this may be related to an increased sequestration of 25-OH-D in fat tissues in obese patients with a large body fat pool, since vitamin D is fat-soluble and is easily stored in adipose tissue; its release into the blood is limited. The negative correlations between the alterations in serum 25-OH-D levels and changes in anthropometric indices such as body weight, BMI, WHR, and total body fat mass in our cohort may have therefore been related to the decline in accumulated 25-OH-D in adipose tissue, with increased release into the blood on reduction of body fat.
Tzotzas T et al (2010) observed that increased levels of 25-OH-D after 10% weight loss following a calorie-restricted diet remarkably correlated with a reduction in insulin levels and the HOMA-IR index. Our study was too short to derive a similar correlation between 25-OH-D and insulin resistance. Similarly, long-term dietary intervention studies have reported that adiponectin concentrations in the blood increase following weight reduction (Golubović MV et al 2013; Harvey AE et al 2013). However, we could not observe a similar effect during our short dietary intervention. We observed that increased 25-OH-D levels were significantly linked to decreased serum IGF-1 and TG levels, relative to the degree of weight loss. This suggests that vitamin D might inhibit IGF-1-induced endothelial cell hyperproliferation and improve unfavorable blood lipid profiles associated with arteriosclerosis.
SUMMARY AND CONCLUSION
This study investigated the impact of weight loss induced by a VLCD on serum 25-OH-D levels, and the associated insulin resistance and inflammation related to cardiovascular disease. The VLCD program extended for 11 days, with participants consuming less than 500 kcal per day. We analyzed serum 25-OH-D levels and biochemical indices including serum lipid profiles, fasting glucose, insulin, IGF-1, HOMA-IR, HbA1, adipokines, and inflammatory biomarkers after dividing the participants into 4 groups according to the quartile of the degree of weight loss (Q1: highest weight loss of ≥3.2 kg, Q2: second highest weight loss of 1.7 kg to <3.2 kg, Q3: third highest weight loss of 0.7 kg to <1.7 kg, Q4: lowest weight loss of <0.7 kg). The results are summarized as follows:
- 1. The participants were 84 pre-menopausal women with an average age of 24.2±7.5 years. General characteristics, including occupation, educational level, and marital status, did not significantly differ between the quartile groups categorized by degree of weight loss. Compared to the other quartile groups, the proportion of participants considering themselves obese was significantly higher in the highest weight loss group (Q1) than in the other weight loss groups. Other factors associated with obesity, such as previous experience with weight loss programs, did not differ between the quartile groups.
- 2. The average percentage of weight loss in the Q1 group was 5.7%, which was significantly higher than that in the other groups. The average percentage of weight loss in the Q2, Q3, and Q4 groups was 3.8%, 1.8%, and 0.3%, respectively. The observed decrease in BMI, WHR, and trunk and total fat masses in the Q1 group were significantly higher than that of the Q3 and Q4 groups, with no significant differences in the changes of waist circumference and percentage of body fat between the groups. The average t-score significantly increased in all weight loss groups except for Q4 group, and the weight-loss-induced increases in the BMD of vertebrae 1∼4 of the lumbar spine were significantly higher in Q1, Q2, and Q3 groups than in Q4 group.
- 3. Q1 group demonstrated an increase in serum 25-OH-D levels after reduction of body weight. Conversely, the 25-OH-D concentrations in the other groups were reduced after the trial. Dietary vitamin D intake decreased in all groups, but the changes were not significantly different between the groups.
- 4. Compared to the other groups, after adjustment for changes in vitamin D intake, the serum 25-OH-D after intervention was significantly increased in Q1 group, with the highest weight loss. Q2 group, which lost more weight than Q3 group, showed a significant decline in serum 25-OH-D levels compared to the latter.
- 5. In groups Q1 and Q2, the serum glucose, insulin, HbA1c, and HOMA-IR score were significantly lowered with reductions in body weight; the reduction in Q1 group was significantly higher than that in Q4 group. The serum levels of leptin and IGF-1 significantly decreased after dietary restriction in groups Q1 (p<0.001) and Q2 (p<0.001), and except for Q4 group, the serum adiponectin levels significantly decreased in all groups, with a significantly higher decrease observed in Q1 group than in the other groups.
- 6. The levels of serum total cholesterol (p<0.001), TG (p<0.01), HDL-C (p<0.001), and LDL-C (p<0.01) significantly decreased in Q1 group, and free fatty acid concentrations increased in all 4 groups after dietary restriction. Serum homocysteine levels increased after dietary restriction in groups Q1 and Q2. Serum hs-CRP levels were not significantly altered by weight loss in any group.
- 7. Changes in serum 25-OH-D levels significantly negatively correlated with changes in body weight, BMI, WHR, waist circumference, total body fat mass, and serum levels of IGF-1, adiponectin, TG, and HDL-cholesterol, but correlated positively with changes in serum homocysteine and uric acid levels after the reduction of body weight, induced by VLCD. These trends persisted after statistical adjustment for changes in vitamin D intake.
In conclusion, the most significant findings from our study were the negative correlation between alterations in serum 25-OH-D levels and changes in body weight, and elevations in serum 25-OH-D levels after weight loss of 5% or more. Vitamin D has been proposed to be an important nutrient that plays a role in metabolic pathways linked to chronic diseases such as diabetes and cardiovascular disease (Pilz S et al 2012). The results from our cohort show that VLCD-induced weight loss of 5% or more not only increases serum 25-OH-D levels in overweight individuals but may also reduce their risk of these diseases. Nevertheless, the duration of our study was too short to achieve a degree of weight loss that would effectively improve insulin resistance. In addition, the VLCDs resulted in an unexpected increase in homocysteine levels and a decrease in HDL-C due to insufficient intake of vitamins and other nutrients. To evaluate the relationship between elevated serum 25-OH-D levels and risk factors for diabetes and cardiovascular disease, long-term trials are required using VLCDs with high nutritional density and an adequate supply of vitamins and protein that may achieve a 10% reduction in body weight.
The scope of this study has been limited by the lack of a significant correlation between oral vitamin D intake and serum 25-OH-D concentrations. This information could not be derived because vitamin D intake was assessed using the 24-hour diet recall method, that entirely depends on the accuracy of recollection of the participants. Furthermore, we did not collect information related to outdoor activities or hours of exposure to daylight. These limitations necessitate further analysis of the relationship between serum 25-OH-D and vitamin D consumption by considering the levels of activity and by estimating actual vitamin D intakes.
Acknowledgments
This study was supported by a research grant from the Seoul Women’s University (2019, Seoul, Korea). The authors declare that there are no potential conflicts of interest.
References
-
Anderson, JL, May, HT, Horne, BD, Bair, TL, Hall, NL, Carlquist, JF, Lappé, DL, Muhlestein, JB, Intermountain Heart Collaborative (IHC) Study Group, (2010), Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population, Am J Cardiol, 106(7), p963-968.
[https://doi.org/10.1016/j.amjcard.2010.05.027]

-
Baker, S, Jerums, G, Proietto, J, (2009), Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes, Diabetes Res Clin Pract, 85(3), p235-242.
[https://doi.org/10.1016/j.diabres.2009.06.002]

-
Beck, B, Bossenmeyer-Pourié, C, Jeannesson, E, Richy, S, Guéant, JL, (2012), Increased homocysteinemia is associated with beneficial effects on body weight after long-term high-protein, low-fat diet in rats, Nutrition, 28(9), p932-936.
[https://doi.org/10.1016/j.nut.2011.12.015]

- Beyer, KA, (2006), The Lemon Detox Diet: Rejuvenation Sensation, PNP Ltd, Grantham, UK.
-
Blum, M, Dallal, GE, Dawson-Hughes, B, (2008), Body size and serum 25-hydroxyvitamin D response to oral supplements in healthy older adults, J Am Coll Nutr, 27(2), p274-279.
[https://doi.org/10.1080/07315724.2008.10719700]

-
Brandenburg, VM, Vervloet, MG, Marx, N, (2012), The role of vitamin D in cardiovascular disease: From present evidence to future perspectives, Atherosclerosis, 225(2), p253-263.
[https://doi.org/10.1016/j.atherosclerosis.2012.08.005]

-
Broussard, JL, Nelson, MD, Kolka, CM, Bediako, IA, Paszkiewicz, RL, Smith, L, Szczepaniak, EW, Stefanovski, D, Szczepaniak, LS, Bergman, RN, (2016), Rapid development of cardiac dysfunction in a canine model of insulin resistance and moderate obesity, Diabetologia, 59(1), p197-207.
[https://doi.org/10.1007/s00125-015-3767-5]

-
Carlin, AM, Rao, DS, Meslemani, AM, Genaw, JA, Parikh, NJ, Levy, S, Bhan, A, Talpos, GB, (2006), Prevalence of vitamin D depletion among morbidly obese patients seeking gastric bypass surgery, Surg Obes Relat Dis, 2(2), p98-103.
[https://doi.org/10.1016/j.soard.2005.12.001]

-
Choi, HS, Oh, HJ, Choi, H, Choi, WH, Kim, JG, Kim, KM, Kim, KJ, Rhee, Y, Lim, SK, (2011), Vitamin D insufficiency in Korea--a greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHA NES) 2008, J Clin Endocrinol Metab, 96(3), p643-651.
[https://doi.org/10.1210/jc.2010-2133]

- Czeglédi, E, (2017), Motivation for weight loss among weight loss treatment participants, Orv Hetil, 158(49), p1960-1967.
- Delbridge, E, Proietto, J, (2006), State of the science: VLED (very low energy diet) for obesity, Asia Pac J Clin Nutr, 15(Suppl), p49-54.
-
DeLuca, HF, (2004), Overview of general physiologic features and functions of vitamin D, Am J Clin Nutr, 80(6 Suppl), p1689S-1696S.
[https://doi.org/10.1093/ajcn/80.6.1689s]

-
Gannagé-Yared, MH, Chedid, R, Khalife, S, Azzi, E, Zoghbi, F, Halaby, G, (2009), Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population, Eur J Endocrinol, 160(6), p965-971.
[https://doi.org/10.1530/eje-08-0952]

-
Goldner, WS, Stoner, JA, Thompson, J, Taylor, K, Larson, L, Erickson, J, McBride, C, (2008), Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: A comparison with non-obese controls, Obes Surg, 18(2), p145-150.
[https://doi.org/10.1007/s11695-007-9315-8]

- Golubović, MV, Dimić, D, Antić, S, Radenković, S, Djindjić, B, Jovanović, M, (2013), Relationship of adipokine to insulin sensitivity and glycemic regulation in obese women--the effect of body weight reduction by caloric restriction, Vojnosanit Pregl, 70(3), p284-291.
- Gossain, VV, Rao, DS, Carella, MJ, Divine, G, Rovner, DR, (1999), Bone mineral density (BMD) in obesity effect of weight loss, J Med, 30(5-6), p367-376.
- Grant, WB, Holick, MF, (2005), Benefits and requirements of vitamin D for optimal health: A review, Altern Med Rev, 10(2), p94-111.
-
Harvey, AE, Lashinger, LM, Otto, G, Nunez, NP, Hursting, SD, (2013), Decreased systemic IGF-1 in response to calorie restriction modulates murine tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression, Mol Carcinog, 52(12), p997-1006.
[https://doi.org/10.1002/mc.21940]

-
Hatfield, DP, Sweeney, KP, Lau, J, Lichtenstein, AH, (2014), Critical assessment of high-circulation print newspaper coverage of the Institute of medicine report dietary reference intakes for calcium and vitamin D, Public Health Nutr, 17(8), p1868-1876.
[https://doi.org/10.1017/s1368980013002073]

-
Havel, PJ, (2002), Control of energy homeostasis and insulin action by adipocyte hormones: Leptin, acylation stimulating protein, and adiponectin, Curr Opin Lipidol, 13(1), p51-59.
[https://doi.org/10.1097/00041433-200202000-00008]

-
Henning, BF, Tepel, M, Riezler, R, Gillessen, A, Doberauer, C, (1998), Vitamin supplementation during weight reduction--favourable effect on homocysteine metabolism, Res Exp Med (Berl), 198(1), p37-42.
[https://doi.org/10.1007/s004330050087]

-
Henry, RR, Wiest-Kent, TA, Scheaffer, L, Kolterman, OG, Olefsky, JM, (1986), Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects, Diabetes, 35(2), p155-64.
[https://doi.org/10.2337/diab.35.2.155]

-
Huffman, KM, Redman, LM, Landerman, LR, Pieper, CF, Stevens, RD, Muehlbauer, MJ, Wenner, BR, Bain, JR, Kraus, VB, Newgard, CB, Ravussin, E, Kraus, WE, (2012), Caloric restriction alters the metabolic response to a mixed-meal: results from a randomized, controlled trial, PLoS One, 7(4), pe28190.
[https://doi.org/10.1371/journal.pone.0028190]

- Hursting, SD, Switzer, BR, French, JE, Karl, FW, (1993), The growth hormone: Insulin-like growth factor I axis is a mediator of diet restriction-induced inhibition of mononuclear cell leukemia in Fischer rats, Cancer Res, 53(12), p2750-2757.
-
Jang, SY, Lee, JY, Bae, JM, Lee, C, Hong, SN, Kim, A, Kim, HY, (2012), 25-hydroxyvitamin D levels and body mass index in healthy postmenopausal women, Korean J Obstet Gynecol, 55(6), p378-383.
[https://doi.org/10.5468/kjog.2012.55.6.378]

-
Janssen, I, Fortier, A, Hudson, R, Ross, R, (2002), Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women, Diabetes Care, 25(3), p431-438.
[https://doi.org/10.2337/diacare.25.3.431]

- Joesting, JJ, Moon, ML, Gainey, SJ, Tisza, BL, Blevins, NA, Freund, GG, (2014), Fasting induces IL-1 resistance and free-fatty acid-mediated up-regulation of IL-1R2 and IL-1RA, Front Immunol, 9;5, p315.
-
Kardas, F, Kendirci, M, Kurtoglu, S, (2013), Cardiometabolic risk factors related to vitamin D and adiponectin in obese children and adolescents, Int J Endocrinol, 2013, 503270.
[https://doi.org/10.1155/2013/503270]

-
Kim, MJ, Hwang, JH, Ko, HJ, Na, HB, Kim, JH, (2015), Lemon detox diet reduced body fat, insulin resistance, and serum hs-CRP level without hematological changes in overweight Korean women, Nutr Res, 35(5), p409-420.
[https://doi.org/10.1016/j.nutres.2015.04.001]

-
Kim, YJ, Moon, MS, Yang, YJ, Kwon, O, (2012), Relationship between serum 25-hydroxyvitamin D concentration and the risks of metabolic syndrome in premenopausal and postmenopausal women, Korean J Nutr, 45(1), p20-29.
[https://doi.org/10.4163/kjn.2012.45.1.20]

- Korea Ministry of Health & Welfare, (2015), 2015 KDRIs, Dietary Reference Intakes for Koreans, http://www.korea.kr (accessed on 3. 12. 2015).
-
Krikken, JA, Dallinga-Thie, GM, Navis, G, Dullaart, RP, (2012), Short term dietary sodium restriction decreases HDL cholesterol, apolipoprotein A-I and high molecular weight adiponectin in healthy young men: Relationships with renal hemodynamics and RAAS activation, Nutr Metab Cardiovasc Dis, 22(1), p35-41.
[https://doi.org/10.1016/j.numecd.2010.03.010]

-
Laaksonen, DE, Kainulainen, S, Rissanen, A, Niskanen, L, (2003), Relationships between changes in abdominal fat distribution and insulin sensitivity during a very low calorie diet in abdominally obese men and women, Nutr Metab Cardiovasc Dis, 13(6), p349-356.
[https://doi.org/10.1016/s0939-4753(03)80003-0]

-
Lim, S, Shin, H, Kim, MJ, Ahn, HY, Kang, SM, Yoon, JW, Choi, SH, Kim, KW, Song, JH, Choi, SI, Chun, EJ, Shin, CS, Park, KS, Jang, HC, (2012), Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: The Korean longitudinal study on health and aging, J Clin Endocrinol Metab, 97(1), p169-178.
[https://doi.org/10.1210/jc.2011-1580]

-
Mason, C, Xiao, L, Imayama, I, Duggan, CR, Bain, C, Foster-Schubert, KE, Kong, A, Campbell, KL, Wang, CY, Neuhouser, ML, Li, L, W Jeffery, R, Robien, K, Alfano, CM, Blackburn, GL, McTiernan, A, (2011), Effects of weight loss on serum vitamin D in postmenopausal women, Am J Clin Nutr, 94(1), p95-103.
[https://doi.org/10.3945/ajcn.111.015552]

- Matthews, DR, Hosker, JP, Rudenski, AS, Naylor, BA, Treacher, DF, Turner, RC, (1985), Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia, 28(7), p412-419.
-
McCarty, MF, (1999), Vegan proteins may reduce risk of cancer, obesity, and cardiovascular disease by promoting increased glucagon activity, Med Hypotheses, 53(6), p459-485.
[https://doi.org/10.1054/mehy.1999.0784]

- Mohammad, T, Farzad, N, Tagie, GM, Ranjbar, K, (2015), The impact of rapid weight loss on the leptin, adiponectin levels, and insulin resistance among adult free style wrestlers, J Sports Med Phys Fitness, 55(7-8), p805-812.
-
Nishizawa, Y, Koyama, H, Shoji, T, Tahara, H, Hagiwara, S, Aratani, H, Nakatsuka, K, Miki, T, Morii, H, (1992), Altered calcium homeostasis accompanying changes of regional bone mineral during a very-low-calorie diet, Am J Clin Nutr, 56(1 Suppl), p265S-267S.
[https://doi.org/10.1093/ajcn/56.1.265s]

-
Parikh, SJ, Edelman, M, Uwaifo, GI, Freedman, RJ, Semega-Janneh, M, Reynolds, J, Yanovski, JA, (2004), The relationship between obesity and serum 1, 25-dihydroxy vitamin D concentrations in healthy adults, J Clin Endocrinol Metab, 89(3), p1196-1199.
[https://doi.org/10.1210/jc.2003-031398]

- Park, JH, Hong, IY, Chung, JW, Choi, HS, (2018), Vitamin D status in South Korean population, Seven-year trend from the KNHANES, Medicine (Baltimore), 97(26), pe11032.
- Pilz, S, Kienreich, K, Tomaschitz, A, Lerchbaum, E, Meinitzer, A, März, W, Zittermann, A, Dekker, JM, (2012), Vitamin D and cardiovascular disease: Update and outlook, Scand J Clin Lab Invest, 72(Suppl 243), p83-91.
-
Ridker, PM, Hennekens, CH, Buring, JE, Rifai, N, (2000), C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women, N Engl J Med, 342(12), p836-843.
[https://doi.org/10.1056/nejm200003233421202]

-
Rock, CL, Emond, JA, Flatt, SW, Heath, DD, Karanja, N, Pakiz, B, Pakiz, B, Sherwood, NE, Thomson, CA, (2012), Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women, Obesity (Silver Spring), 20(11), p2296-2301.
[https://doi.org/10.1038/oby.2012.57]

- Rosen, CJ, (2011), Clinical practice. Vitamin D insufficiency, N Engl J Med, 364(3), p248-254.
-
Schaumberg, K, Anderson, DA, Kirschenbaum, DS, Earleywine, M, (2015), Participation as a leader in immersion weight loss treatment may benefit, not harm, young adult staff members, Clin Obes, 5(4), p226-235.
[https://doi.org/10.1111/cob.12106]

-
Shantavasinkul, PC, Phanachet, P, Puchaiwattananon, O, Chailurkit, LO, Lepananon, T, Chanprasertyotin, S, Ongphiphadhanakul, B, Warodomwichit, D, (2015), Vitamin D status is a determinant of skeletal muscle mass in obesity according to body fat percentage, Nutr J, 31(6), p801-806.
[https://doi.org/10.1016/j.nut.2014.11.011]

- Statistics Korea, (2018), Trend of obesity rate in 2016, http://kosis.kr (accessed on 31. 1. 2018).
-
Tzotzas, T, Papadopoulou, FG, Tziomalos, K, Karras, S, Gastaris, K, Perros, P, Krassas, GE, (2010), Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance, J Clin Endocrinol Metab, 95(9), p4251-4257.
[https://doi.org/10.1210/jc.2010-0757]

-
Willis, CM, Laing, EM, Hall, DB, Hausman, DB, Lewis, RD, (2007), A prospective analysis of plasma 25-hydroxyvitamin D concentrations in white and black prepubertal females in the southeastern United States, Am J Clin Nutr, 85(1), p124-130.
[https://doi.org/10.1093/ajcn/85.1.124]

-
Wortsman, J, Matsuoka, LY, Chen, TC, Lu, Z, Holick, MF, (2000), Decreased bioavailability of vitamin D in obesity, Am J Clin Nutr, 72(3), p690-693.
[https://doi.org/10.1093/ajcn/72.3.690]

- Zoccali, C, Mallamaci, F, Tripe~pi, G, Benedetto, FA, Cutrupi, S, Parlongo, S, Malatino, LS, Bonanno, G, Seminara, G, Rapisarda, F, Fatuzzo, P, Buemi, M, Nicocia, G, Tanaka, S, Ouchi, N, Kihara, S, Funahashi, T, Matsuzawa, Y, (2002), Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease, J Am Soc Nephrol, 13(1), p134-141.
