Optimization of Vitamin D2 Production using Enoki Mushroom Powder and the Preparation of Doenjang using the Powder
Abstract
Optimization of vitamin D2 production in enoki mushroom (Flammulina velutipes) using response surface methodology (RSM), and the effect of adding Enoki mushroom on the general quality characteristics of Doenjang during the fermentation process were studied. For the conversion of ergosterol in enoki mushroom powder to vitamin D2 via ultraviolet-B (UV-B), the three independent variables of exposure time (40∼120 min), ambient temperature (10∼30℃) and irradiation intensity (0.9 ∼2.1 W/m2) were investigated. The ridge analysis predicted that a maximum yield of 393.73 μg/g was obtained when ambient temperature, exposure time, and irradiation intensity were 23.54℃, 82.33 min and 1.11 W/m2, respectively. During the fermentation process, in comparison with the control sample, bacterial and yeast counts, pH value, protease activity, amino-type nitrogen content, vitamin D2 content and 5’-nucleotides contents increased in Doenjang after adding enoki mushroom. Beyond this, the addition of enoki mushroom reduced total acidity and had no effect on reducing sugar content, total amylase or α -amylase activity in Doenjang.
Keywords:
Flammulina velutipes, enoki mushroom, vitamin D2, Doenjang, fermentationINTRODUCTION
Vitamin D, which most people mainly obtain from sunlight exposure, is essential for human health (Koyyalamudi et al. 2009). However, for many reasons, including different ethnicity, seasonal shift, the use of sun screen and obesity, a considerable number of people can’t get enough sunshine exposure to synthesize vitamin D, which leads to a vitamin D insufficiency or deficiency (Koyyalamudi et al. 2009; Wu & Ahn 2014). In this case, dietary supplement is necessary. Anecdotal evidence has suggested that vitamin D is essential for human health, and is closely related to many diseases, including osteoporosis, cardiovascular disease, type 1 diabetes, and some cancers (Holick MF 2004).
Vitamin D, including vitamin D2 and vitamin D3, can only be found in a few kinds of natural food (Shrapnel & Truswell 2009; Jasinghe & Perera 2006). Vitamin D3 is only found in animal sources, such as cod liver oil and oily fish (Moyad MA 2009), while vitamin D2 exists in mushrooms, particularly in mushrooms exposed to UV irradiation, where ergosterol undergoes photolysis to yield a relative quantity of vitamin D2 (Jasinghe & Perera 2005). Although there is still some controversy over whether vitamin D2 is effective in humans, they both produce rises in the serum 25-hydroxyvitamin D level (Armas et al. 2004; Heaney et al. 2011; Holick et al. 2008). In other words, compared with animal sources, mushroom species are healthier and more acceptable sources of vitamin D.
Enoki mushroom (Flammulina velutipes), also known as winter mushroom or golden needle mushroom, is very popular in North America, Europe and Asia, which ranks the fifth in term of global mushroom production (Jasinghe VJ 2005; Tong et al. 2008). This delicious mushroom species is rich in proteins and dietary fiber (20 and 29.3% on a dry basis, respectively) and low in fat and calories (Tong et al. 2008; Dikeman et al. 2005). In addition, it contains fungal immunomodulatory protein (FIP-fve), ribosome inactivating protein (RIP), active polysaccharides and some other bioactive compounds, which help to suppress cancers, human immuno-deficiency virus (HIV) and cardiovascular diseases (Tong et al. 2008; Dikeman et al. 2005; Zhang et al. 2005). Moreover, it is a familiar and cheap source to supply people with enough vitamin D, as the mushroom species is abundant in ergosterol, the pro-vitamin D2 (Mattila et al 2002). For this reason, enoki mushrooms are chosen as a supplement for those who are deficient in vitamin D.
The conversion of ergosterol in mushrooms to vitamin D2 has been well reported (Koyyalamudi et al 2009; Wu & Ahn 2014; Jasinghe & Perera 2005; Jasinghe & Perera 2006); however, little attention has been paid to the changes in the physicochemical properties of food added with UV-irradiated mushrooms. The effect of irradiation sources on vitamin D2 synthesis in mushrooms has been in-depth studied before (Wu & Ahn 2014; Jasinghe & Perera 2006; Jasinghe et al. 2007; Teichmann et al. 2007). In a research conducted by Wu & Ahn (2014), oyster mushrooms were irradiated under UV-A, UV-B and UV-C, respectively. It was proved that UV-B is more effective than UV-A and UV-C on vitamin D2 conversion in mushrooms, which further validated the conclusion of Jasinghe & Perera (2006).
Thus, in this study, the response surface methodology was used to determine the optimum ultraviolet irradiate conditions for the conversion of ergosterol in enoki mushroom powder to vitamin D2. Then, the mushroom powder was added to fortify Doenjang with vitamin D, and the quality characteristics of Doenjang was investigated.
MATERIALS AND METHODS
1. Reagents
Enoki mushrooms were purchased from local growers in Jeonju, Korea. Ergosterol, vitamin D2 (ergocalciferol), Folin & Ciocalteu’s phenol reagent, L-tyrosine, Na-metabisulfite, 3,5-dinitrosalicylic acid 5’-AMP, 5’-CMP, 5’-GMP, 5’-IMP, 5’-UMP and 5’-XMP were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals were of reagent grade. Enoki mushrooms were freeze-dried, ground, and passed through a 50 mesh sieve to obtain the powdered samples.
2. Vitamin D2 Extraction and Analysis
Vitamin D2 in mushrooms and Doenjang was determined according to a previously described method (Wu & Ahn 2014). One g of freeze-dried enoki mushroom powder or 10 g of Doenjang was put in a 250 mL flask, and mixed with 4 mL of sodium ascorbate solution. The mixture was saponified under reflux at 80℃ for 1 h, then extracted sequentially with 15 mL deionized water, 15 mL ethanol, and 50 mL n-pentane (three times). The organic layer was cleaned with 50 mL of 3% KOH in 5% ethanol (two times), then washed with deionized water until neutralized. The organic layer was concentrated by a rotary evaporator, and the dried residue was dissolved in 10 mL ethanol for HPLC analysis. The analysis for vitamin D2 was performed with a Waters Millenium system, equipped with a Waters 600 Controller gradient pump, a Waters 717 Plus autosampler, and a Waters UV-486 detector (Waters, MA). The column used was a SunFire C18 analytical column (2.6 × 250 mm, 5 μm, Waters, Ireland). The mobile phase was methanol/acetonitrile (25:75,v/v). The column temperature was 30℃, and the filtered sample injection volume was 20-μL. The flow rate was 1.0 mL/min, and the eluate was monitored at 280 nm.
3. Optimization for Vitamin D2 Synthesis in Enoki Mushroom Powder using Response Surface Methodology (RSM)
UV-B lamps (Model G15T8E, Sankyo Denki, Japan) were used as the source of irradiation to determine the optimum conditions for vitamin D2 synthesis in this study. The effects of ambient temperature, exposure time, and irradiation intensity for vitamin D2 synthesis in enoki mushroom powder were examined in a preliminary experiment. The results of the preliminary experiments are shown in Table 1. The three independent variables of ambient temperature (A), exposure time (B) and irradiation intensity (C) were used to optimize the vitamin D2 yield using RSM based on the preliminary experiment. The range and levels of the independent variables investigated in this study are indicated in Table 2. To describe the relationships between the process index (the yield of vitamin D2) and the three irradiate factors, a mathematical second-order equation was established. The yield of vitamin D2 was analyzed by multiply regressions using the least squares method as follows:
where, y is the predicted response, xi and xj are the coded independent variables, b0 is aconstant, bi is the linear coefficient, and bij is the interaction coefficient. The accuracy and general ability of the above polynomial model were evaluated using the coefficient of determination R2.

Effects of independent variables on vitamin D2 synthesis in enoki mushroom powder under UV-Birradiation
Design-Expert Software (Version 8.0.6, Stat-Ease Inc) was used to analyze and process the second-order polynomial coefficients, and the model was validated for the experimental conditions used in this study. The combinations of the three independent variables together with the responses are shown in Table 3. The response measured was vitamin D2 content.
4. Preparation of Doenjang and Analysis
Meju was purchased from Sunchang Meju Corporation (Sunchang, Korea). Three kg of meju powder (99.9% soybean, 0.1% meju strain), 1.8 L (20 brix) salt brine and UV-B irradiated mushroom powder (0%, 1%, 2%, and 4%) were prepared and mixed. Doenjang samples with 0%, 1%, 2%, and 4% of mushroom added were denoted as A0, A1, A2 and A3, respectively. Fermentation of each sample proceeded for 3 months at room temperature (25±2℃), and the samples were analyzed at regular intervals (0, 1, 2, 3 months).
A 10 g sample of Doenjang was mixed with 90 mL of saline solution, then stirred for 30 min at room temperature, and serially diluted 10× with saline solution. Each diluent (0.1 mL) was poured in triplicate on each selective agar plate. Media for enumeration of yeast was prepared by adding chloramphenicol (100 mg/L) to potato dextrose agar (Becton, Dickinson and Company, MD, USA). Selective media used for bacteria was plate count agar (Becton, Dickinson and Company, MD, USA). Yeasts were incubated for three days and bacteria were cultured overnight. The incubation temperatures for yeast and bacteria were 25℃ and 37℃, respectively.
Ten g of Doenjang was mixed with 10 mL deionized water and the pH of Doenjang was determined using a pH meter (SevenEasy S20, Mettler-Toledo GmbH, Schwerzenbach, Switzerland). To measure the total acidity, a 5 g sample was mixed with 50 mL deionized water and then diluted to a 100 mL volume. Twenty mL of the mixture was then transferred to a flask and titrated with 0.05 N sodium hydroxide to an endpoint of pH 8.2 and total acidity appeared by lactic acid contents.
Ten g of Doenjang was mixed with 90 mL of deionized water and stirred for 30 min at room temperature, then was filtered through a Whatman No.3 filter paper. Neutral protease activity was measured using the previous method described by Jung et al. (1994) with slight modification. One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 μmol tyrosine per minute. Optical density of each filtrate was determined using a spectrophotometer (UV 1650PC, Shimadzu, Tokyo, Japan) at wavelength of 660 nm. α-Amylase activity and total amylase activity were determined based on the method of Li et al. (2005) with some modification. The absorbance of each sample solution was measured at 540 nm against a blank of deionized water at Shimadzu UV-1650 spectrophotometer. Amylase activity was calculated based on a calibration curve of maltose.
The reducing sugar in Doenjang was determined using the dinitrosalicylic acid (DNS) method. In this method, glucose was chosen as the standard, prepared in concentrations of 0 mg/mL, 0.4 mg/mL, 0.8 mg/mL, 1.2 mg/mL, 1.6 mg/mL and 2.0 mg/mL. A 5 g sample was mixed with 50 mL deionized water and stirred for 30 min at room temperature, then filtered through a Whatman No.3 filter paper. One milliliter of standard solution or filtrate was pipetted into a test tube and 3 mL of DNS reagent was added to it. The mixture was boiled for 5 min and then cooled to room temperature under running water. OD540 was measured with a blank of deionized water.
Five g of Doenjang was mixed with 50 mL deionized water and stirred for 30 min at room temperature. It was then diluted to a 100 mL volume. Twenty mL of the mixture was then transferred to a flask and titrated with 0.05 N sodium hydroxide to an endpoint of pH 8.2. Ten mL of neutral formalin was added and the titration was repeated, using a pH meter to reach pH 9.2. The control group was treated in the same manner, except that deionized water was used instead of the sample.
The free amino-type nitrogen in Doenjang was calculated using the following equation.
Where X is the free amino-type nitrogen content (g/g), V1 and V2 are the titrant volume of sodium hydroxide that of the blank and sample, consumed from adding formaldehyde to the endpoint of pH 9.2.
5’-nucleotides were extracted and analyzed as described by Taylor et al.(2005) with slight modification. A mixture of Doenjang and deionized water (10 g sample mixed with 25 mL deionized water) was boiled for 10 min, cooled, and then centrifuged at 12,000 rpm for 15 min. The extraction was repeated with 20 mL deionized water, the combined supernatants were evaporated to dryness and filtered through a 0.22-μm cellulose membrane (Chromdisc, Daegu, Korea) prior to analysis by high-performance liquid chromatography(HPLC) using a Waters Alliance e2695 instrument (Waters Corporation, MA, USA) equipped with a Sunfire C18 column (4.6 × 250 mm, 5 μm, Waters, Ireland). HPLC conditions were as follows: mobile phase, 0.05 N K2HPO4/H3PO4 (pH 4.0); flowrate, 1.0 mL/min; UV detection wavelength, 254 nm; oven temperature, 25℃; injection volume, 20 μL. Each 5’-nucleotide was identified and quantified by using 5’-nucleotide standards.
RESULTS AND DISCUSSION
1. Selections of Irradiation Conditions for Vitamin D2 Synthesis in Enoki Mushroom Powder
UV-B was selected as the irradiation source in this study. Additionally, the effects of three irradiation factors including ambient temperature, irradiation intensity and exposure time on vitamin D2 conversion were also in vestigated and shown in Table 1. Vitamin D2 yield in enoki mushrooms reached a peak after being irradiated under UV-B for 80 min (1.2 W/m2, 30℃). As well, vitamin D2 level produced at 1.5 W/m2 (80 min, 30℃) was higher than those at other intensities. In terms of temperature, definitely, 20℃ was the best for vitamin D2 conversion in enoki mushrooms. Therefore, the range and levels chosen for the Central Composite Rotatable Design were tabulated (Table 2).
2. Optimization of the Key Factors for Vitamin D2 Synthesis in Enoki Mushroom Powder
A Central Composite Rotatable Design (CCRD) was conducted to further explore the effects of the three factors on vitamin D2 synthesis. After applying multiple regression analysis on the experimental data presented in Table 3, a second-order polynomial model equation was obtained to describe the vitamin D2 synthesis as follows:
where, Y is the predicted vitamin D2 content (μg/g, dry-weight); A (ambient temperature), B (exposure time) and C (irradiation intensity) are the coded values.
The experimental data on related statistics was evaluated through ANOVA (Table 4). The model F-value of 27.14 implied that the model was significant. There was only a 0.01% chance that this large F value could occur due to noise. P-values in this study less than 0.05 indicate model terms are significant. In this case, C, AB, AC, BC, A2, B2, and C2 are significant model terms. The R2 value was 96.07%, which meant that the model could account for 96.07% of the variability. It indicates a good agreement between experimental results and predicted values and the proposed model means that it is reliable for vitamin D2 synthesis in this study.
Response surface plots and contour plots were obtained based on the proposed model (Fig. 1A to Fig. 1C). Fig. 1A depicts the effects of interactions of ambient temperature (10 to 30℃) and exposure time (40 to 120 min) with a fixed irradiation intensity of 1.5 W/m2. Factor ambient temperature gave rise to a linear increase effect on the response under low levels of ambient temperature when the irradiation intensity was maintained at 1.5 W/m2. In addition, the maximum value was obtained between 18℃ to 22℃. Both the ambient temperature and irradiation intensity demonstrated quadratic effects on the response, as is shown in Fig. 1B. Fig. 1C depicts the interactions of exposure time and irradiation intensity of the response, and showed a similar trend between two variables. Tanyildizi et al. (2005) demonstrated that the maximum predicted value is indicated by the surface confined in the smallest ellipse in the contour diagram. Based on the analysis with Design expert, the model predicted a maximum vitamin D2 content of 393.73 μg/g, with a 95% confidence interval between 382.49 μg/g and 404.97 μg/g, under the condition of ambient temperature, exposure time and irradiation intensity were 23.54℃, 82.33 min, 1.11 W/m2, respectively.
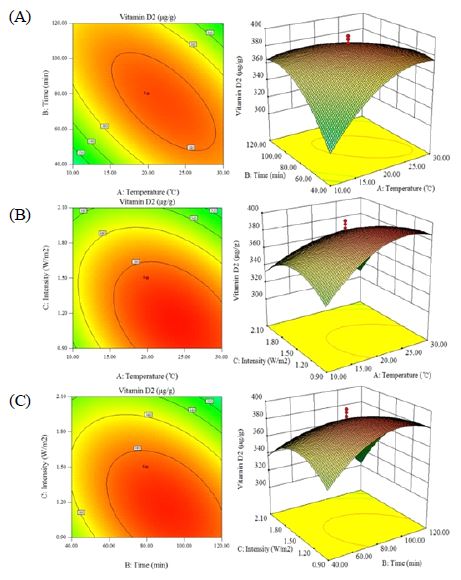
Contour and 3D response surface plots showing the effects of (A) interaction between ambient temperature and exposure time on vitamin D2 synthesis in enoki mushroom powder, with irradiation intensity of 1.5 W/m2; (B) interaction between ambient temperature and irradiation intensity on vitamin D2 synthesis in enoki mushroom powder, with exposure time of 80 min; (C) interaction between exposure time and irradiation intensity on vitamin D2 synthesis in enoki mushroom powder, with ambient temperature of 20℃.
3. Model Verification
Under the above optimum conditions, a vitamin D2 level of 391.57±13.25 μg/g was produced in enoki mushroom powder, within 95% confidence of the predicted maximum value (393.73 μg/g). The excellent correlation of the predicted and measured values verifies the utility and practicability of the proposed model. Moreover, no other nutritionally or toxicologically significant changes in mushroom composition are produced during ultraviolet irradiation (Simon et al. 2011; Zhang & Ahn 2015), which indicates that these parameters could be applied to the following research. Chio et al. (2005) reported vitamin D2 levels of mushrooms. Among the mushrooms, the content of vitamin D2 in enoki mushroom was about 5.7 μg/g; the conversion of vitamin D by UV irradiation was not carried out. However, through the present study, vitamin D in enoki mushroom was increased up to about 391 μg/g by UV irradiation.
4. Quality Characteristics of Doenjang prepared with Enoki Mushroom Powder
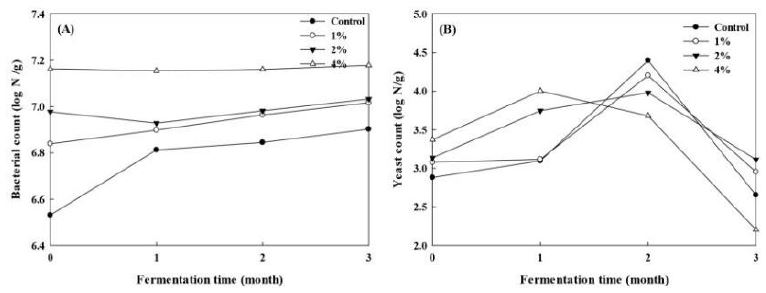
Bacteria are involved as the dominant strains, particularly bacilli, during the Doenjang fermentation, and fungi and yeasts are detected as the predominant (Kim et al. 2009, Jeong et al. 2014). However, the predominant yeasts play an important role in the flavor and taste formation of Doenjang (Haque et al. 2015). Therefore, the survival rate and change in the growth of bacteria (Fig. 2A) and yeast (Fig. 2B) in Doenjang during fermentation was measured. At the initial stage of fermentation, the number of bacteria was 106 cells/g in A0 group, but this increased to 107 cells/g after adding 4% enoki mushroom powder. As the fermentation continued, bacteria count increased significantly in A0 and A1 groups, with a final level of 0.8 × 107 and 1.04 × 107 cells/g. In A2 and A3 groups, bacterial population was maintained at about 1.00 × 107 and 1.45 × 107 cells/g throughout the whole fermentation, respectively. For the growth of yeast in Doenjang, the addition of mushroom increased the yeast number in samples at the start of fermentation. This could be attributed to the fact that UV-B irradiation didn’t eliminate all fungus in enoki mushroom. With the fermentation on, the yeast count increased sharply first in A4 group, as the surrounding environment of microorganisms was richer in the substances needed for yeast growth. The yeast count in the other three samples reached a peak after 2 months’ fermentation and then declined dramatically to a stable value of about 103 /g.
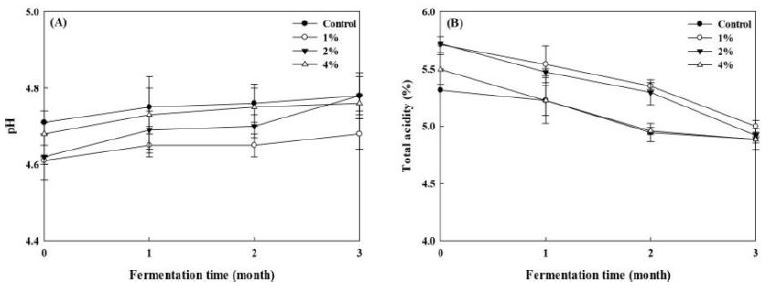
As was described in Fig. 3A, Doenjang fortified with mushroom powder showed no difference in pH values in comparison with the control group. In addition, the pH of all the samples tended to increase slightly after 3 months’ fermentation. Correspondingly, there was a certain decrease in the total acidity of each group (Fig. 3B), with the smallest of 0.42% in A0 and the largest of 0.80% in A2. There are two main factors associated with pH and total acids during the fermentation of Doenjang. On the one hand, lipids and carbohydrates are broken down by microorganisms into low-molecular-weight organic acids, like acetic acid and lactic acid, which results in the decline of pH and the increase of total acids content. On the other hand, these acids combine with the alcohols generated by the yeasts and produce neutral esters. These may explain the changes of pH and total acidity in Doenjang samples.
The changes of neutral protease activity in Doenjang during storage are shown in Fig. 4. At the initial stage of fermentation, the activity value was between 1.73 to 1.79 unit/g in all the samples. The enzymatic activity in A0 group decreased sharply to 1.54 unit/g after 1 month’s storage and then gradually declined to 1.44 unit/g in the following 2 months. In Doenjang fortified with enoki mushroom, the activity of all the three groups dropped slowly in the first two months and rapidly in the last to a final level between 1.45 to 1.48 unit/g.
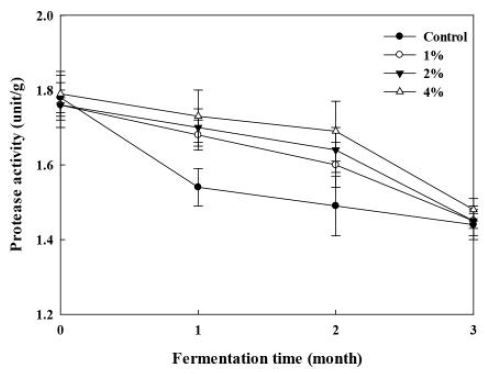
Changes of protease activity in Doenjang added with different percentage of enoki mushroom powder during fermentation at room temperature.
Similar to protease activity, both α-amylase and total amylase activity declined clearly during 3 months’ storage (Table 5). The change in amylase activity of Doenjang according to fermentation time showed no significant difference in each treatment, regardless of whether it was fortified with enoki mushroom or not. The main difference between the changes of α-amylase and total amylase activity was the different descent rate. Total amylase activity declined sharply by up to 50%. On the other hand, α-amylase activity experienced a gentler decline, with a decrease rate of about 20%. Enzymatic activity is closely related with environmental temperature, pH, ion content and storage time. Thus, the constant value of enzymatic activity in turn indicates that the fermentation of Doenjang has reached a relative equilibrium after 3 months of fermentation. Rhee et al. (2000) investigated enzyme activity in the traditional Deonjang with various concentration of Lentinus edodes during fermentation. Amylase and protease activities gradually decreased as the fermentation progressed. In traditional Deonjang with increasing the mixture ratio Lentinus edodes, amylase and protease activities were decreased and increased, respectively. Overall, amylase and protease activities tended to be similar to those in traditional Deonjang with Lentinus edodes in this study. However, there were no significant differences in amylase activity according to the Enoki mushroom powder concentrations between the samples and the control (Deonjang without the enoki mushroom powder).
Generally, reducing sugar content reaches its maximum within one to two weeks’ fermentation in soybean paste, because glucoamylase and amylase, generated by fungi, catalyze the hydrolysis of starch in sauce into reducing sugar rapidly, increasing the reducing sugar content afterwards. As the fermentation continues, reducing sugar content decreases due to microbial consumption or utilization. On the one hand, reducing sugar is a significant carbon source for microbial growth. On the other, it is also necessary for the Maillard reaction. Table 6 depicted the changes of reducing sugar content in Doenjang during storage. There were no changes in reducing sugar content in terms of the addition of enoki mushroom. At the start of the fermentation, the reducing sugar level was about 3.2%, and increased to a peak of about 4.3% within 2 weeks’ storage. Then it dropped gradually and maintained at a level of about 2.2%.
The amino-type nitrogen content is one of the important indicators of the successful fermentation of Doenjang (Kim & Rhyu 2000). During fermentation, proteins in Doenjang are degraded into peptides and amino acids, bringing about an increase in amino-type nitrogen. Table 6 showed the changes of amino-type nitrogen content in Doenjang during the fermentation. Amino-type nitrogen tended to increase in all of the 4 samples. It increased rapidly in the first month and more slowly in the following period, since the protease activity was higher at the initial stage of the fermentation, catalyzing the hydrolysis of proteins more efficiently. As the fermentation time got longer, the protease activity went down gradually for the changes of surrounding environment. In addition, easily degradable proteins were nearly exhausted and the persistent proteins were degraded slowly. After 3 months’ fermentation, amino-type nitrogen reached a level of about 0.9% (900 μg/g) in all the 4 samples. Choi et al. (2006) reported that the contents of amino-type nitrogen in the traditional Deonjangs with added Lentinus edodes increased as Lentinus edodes was added higher. In this study, there were no significant differences in amino-type nitrogen contents according to the enoki mushroom powder concentrations between the samples and the control before fermentation. However, amino-type nitrogen contents of the samples gradually increased as the fermentation progressed.
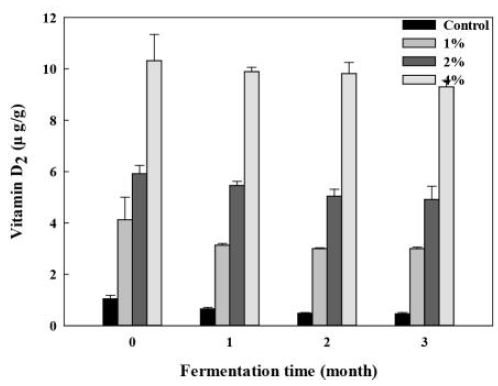
The changes of vitamin D2 content in Doenjang added with different percentages of Enoki mushroom were shown in Fig. 5. The control group also contained a little amount of vitamin D2 (1.04 μg/g), as there was vitamin D2 in meju (2.56 μg/g, measured in a preliminary experiment), a main ingredient of Doenjang. The vitamin D2 level in Doenjang fortified with enoki mushroom was much higher than that in A0 group, from 4.12 μg/g in A1 sample to 10.32 μg/g in A3 sample. During 3 months’ storage, vitamin D2 content gradually decreased, at a rate of 55.8%, 27.2%, 17.1%, and 9.9% in Doenjang with 0%, 1%, 2%, and 4% mushroom added, respectively.
Flavor 5’-nucleotides, including 5’-GMP, 5’-IMP and 5’-XMP, are responsible for the umami or palatable taste. In particular, 5’-GMP is a flavor enhancer much stronger than MSG, generating a meaty flavor (Litchfield 1967). As was shown in Table 7, flavor 5’-nucleotides in Doenjang increased obviously with the addition of enoki mushroom in a dose-dependent manner, ranging from 38.30 in the control group to 229.70 μg/g in Doenjang fortified with 4% enoki mushrooms. As the fermentation continued, among the three flavor 5’-nucleotides, 5’-IMP increased slightly. At the same time, 5’-GMP and 5’-XMP decreased at different levels during the 3 months’ storage. In general, flavor 5’-nucleotdies first decreased and finally maintained at a certain level. In the traditional Deonjang with added Lentinus edodes, the contents of nucleic acid related substances increased as Lentinus edodes was added higher. The contents of GMP and IMP, which significantly affect the taste of soybean paste, were found to be as high as in this study (Choi et al. 2006).
SUMMARY
Vitamin D is produced by the body as a response to sun exposure; it can also be consumed in food or supplements. Although the body can create vitamin D, there are many reasons deficiency can occur. Therefore, it is best to intake vitamin D from natural foods; however, their contents are low. UV irradiation is able to increase the vitamin D level in food raw materials. Among the natural substances, mushrooms are very rich in ergosterol, a precursor of vitamin D, and can easily be converted from ergosterol to vitamin D2 by UV irradiation. The results of the present study revealed that ambient temperature, exposure time, and UV-B irradiation intensity are significant factors associated with the vitamin D2 synthesis in enoki mushrooms. Based on a central composite rotatable design (CCRD), a maximum vitamin D2 yield of 393.73 μg/g was obtained when ambient temperature, exposure time and irradiation intensity were 23.54℃, 82.33 min and 1.11 W/m2, respectively. However, there was still remained a large amount of vitamin D2 precursor ergosterol in UV-irradiated Enoki mushroom powder. Hence, further studies are needed to convert ergosterol into vitamin D completely. During fermentation, compared with the control group, protease activity, amino-type nitrogen, and 5’-ncleotides contents increased differently in Doenjang fortified with UV-irradiated enoki mushroom powder. It is notable that the addition of enoki mushroom gave Doenjang a relatively high level of vitamin D2, 10.32 μg/g in the sample with 4% Enoki mushroom powder added. Therefore, the Doenjang with relatively high levels of vitamin D in this study, is presumed to be a good food source for those who are vitamin D deficient. Also, enoki mushroom powder with high level of vitamin D2 could be used for various soybean-fermented foods such as soy sauce, red pepper paste, and Cheonggukjang.
Acknowledgments
This work (Grant No. C0249145) was supported by Business for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2014.
REFERENCES
-
Armas, LAG, Hollis, BW, Heaney, RP, (2004), Vitamin D2 is much less effective than vitamin D3 in humans, J Clin Endocrinol Metab, 89(11), p5387-5391.
[https://doi.org/10.1210/jc.2004-0360]

- Choi, HS, Kim, MK, Kim, MK, Park, HS, Song, GS, Lee, KK, Kim, TY, Kim, JG, (2005), An approach to increase vitamin D2 level in Doenjang (fermented soybean paste) using mushrooms, Food Sci Biotechnol, 14(6), p828-831.
- Choi, SY, Sung, NJ, Kim, HJ, (2006), Physicochemical characteristics of traditional Doenjang with added Lentinus edodes, Korean J Food Cookery Sci, 22(1), p69-79.
-
Dikeman, CL, Bauer, LL, Flickinger, EA, Fathy, GC, (2005), Effects of stage of maturity and cooking on the chemical composition of select mushroom varieties, J Agric Food Chem, 53(4), p1130-1138.
[https://doi.org/10.1021/jf048541l]

-
Haque, MA, Seo, WT, Hwang, CE, Lee, HY, Ahn, MJ, Cho, KM, (2015), Culture-independent analysis of yeast diversity in Korean traditional fermented soybean foods (doenjang and kanjang) based on 26S rRNA sequence, J Korean Soc Appl Biol Chem, 58(3), p377-385.
[https://doi.org/10.1007/s13765-015-0030-1]

- Heaney, RP, Recher, RR, Grote, J, Horst, RL, Armas, LAG, (2011), Vitamin D3 is more potent than vitamin D2 in humans, J Clin Endocrinol Metab, 96(3), p447-452.
-
Holick, MF, (2004), Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis, Am J Clin Nutr, 79(3), p362-371.
[https://doi.org/10.1093/ajcn/79.3.362]

- Holick, MF, Biancuzzo, RM, Chen, TC, Klein, EK, Young, A, Bibuld, D, Reitz, R, Salameh, W, Ameri, A, Tannenbaum, AD, (2008), Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D, J Clin Endocrinol Metab, 93(3), p677-684.
- Jasinghe, VJ, (2005), Conversion of ergosterol in edible mushrooms to vitamin D2 by UV irradiation, Ph D Dissertation, Singapore National University, Singapore, p21.
-
Jasinghe, VJ, Perera, CO, (2005), Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation, Food Chem, 92(3), p541-546.
[https://doi.org/10.1016/j.foodchem.2004.08.022]

-
Jasinghe, VJ, Perera, CO, (2006), Ultraviolet irradiation: The generator of vitamin D2 in edible mushrooms, Food Chem, 95(4), p638-643.
[https://doi.org/10.1016/j.foodchem.2005.01.046]

-
Jasinghe, VJ, Perera, CO, Sablani, SS, (2007), Kinetics of the conversion of ergosterol in edible mushrooms, J Food Eng, 79(3), p864-869.
[https://doi.org/10.1016/j.jfoodeng.2006.01.085]

-
Jeong, DW, Kim, HR, Jung, G, Han, S, Kim, CT, Lee, JH, (2014), Bacterial community migration in the ripening of doenjang, a traditional Korean fermented soybean food, Microbiol Biotechnol, 24(5), p648-660.
[https://doi.org/10.4014/jmb.1401.01009]

- Jung, SW, Kim, YH, Koo, MS, Shin, DB, Chung, KS, Kim, YS, (1994), Changes in physicochemical properties of industry-type Kochujang during Storage, Korean J Food Sci Technol, 26(4), p403-410.
- Kim, EY, Rhyu, MR, (2000), The chemical properties of Doenjang prepared by Monascus koji, Korean J Food Sci Technol, 32(5), p1114-1121.
-
Kim, TW, Lee, JH, Kim, SE, Park, MH, Chang, HC, Kim, HY, (2009), Analysis of microbial communities in doenjang, a Korean fermented soybean paste, using nested PCR-denaturing gradient gel electrophoresis, Int J Food Microbiol, 131(2-3), p265-271.
[https://doi.org/10.1016/j.ijfoodmicro.2009.03.001]

-
Koyyalamudi, SR, Jeong, SC, Song, CH, Cho, KY, Pang, G, (2009), Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation, J Agric Food Chem, 57(8), p3351-3355.
[https://doi.org/10.1021/jf803908q]

-
Litchfield, JH, (1967), Morel mushroom mycelium as a food flavoring material, Biotechnol Bioeng, 9(3), p289-304.
[https://doi.org/10.1002/bit.260090303]

- Li, W, Shao, YZ, Chen, WX, (2005), Improved method for determining amylase activity, Plant Physiol Comm, 41(5), p655-656.
-
Mattila, PH, Lampi, AM, Ronkainen, R, Toivo, J, Piironen, V, (2002), Sterol and vitamin D2 contents in some wild and cultivated mushrooms, Food Chem, 76(3), p293-298.
[https://doi.org/10.1016/s0308-8146(01)00275-8]

- Moyad, MA, (2008), Vitamin D: a rabid review, Urol Nurs, 28(5), p343-349.
- Rhee, CH, Lee, JB, Jang, SM, (2000), Changes of microorganisms, enzyme activity and physiological functionality in the traditional Deonjang with various concentrations of Lentinus edodes during fermentation, J Korean Soc Agric Chem Biotechnol, 43(4), p277-284.
-
Shrapnel, W, Truswell, S, (2006), Vitamin D deficiency in Australia and New Zealand: what are the dietary options?, Nutr Diet, 63(4), p206-212.
[https://doi.org/10.1111/j.1747-0080.2006.00080.x]

-
Simon, RR, Phillips, KM, Horst, RL, Munro, IC, (2011), Vitamin D mushrooms: comparison of the composition of button mushrooms (Agaricus bisporus) treated postharvest with UVB light or sunlight, J Agric Food Chem, 59(16), p8724-8732.
[https://doi.org/10.1021/jf201255b]

-
Tanyildizi, MS, Ozer, D, Elibol, M, (2005), Optimization of α-amylase production by Bacillus sp. using response surface methodology, Process Biochem, 40(7), p2291-2296.
[https://doi.org/10.1016/j.procbio.2004.06.018]

-
Taylor, MW, Hershey, RA, Levine, RA, Coy, K, Olivelle, S, (1981), Improved method of resolving nucleotides by reverse-phase high performance liquid chromatography, J Chromatogr A, 219(1), p133-139.
[https://doi.org/10.1016/s0021-9673(00)80584-1]

- Teichmann, A, Dutta, PC, Staffas, A, Jagerstad, M, (2007), Sterol and vitamin D2 concentrations in cultivated and wild mushrooms: effect of UV irradiation, LWT, 40(5), p815-822.
-
Tong, MH, Chien, PJ, Chang, HH, Tsai, MJ, Sheu, F, (2008), High processing tolerances of immunomodulatory proteins in enoki and reishi mushrooms, J Agric Food Chem, 56(9), p3160-3166.
[https://doi.org/10.1021/jf800205g]

-
Wu, WJ, Ahn, BY, (2014), Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) using response surface methodology, PLoS One, 9(4), pe95359.
[https://doi.org/10.1371/journal.pone.0095359]

- Zhang, C, Cao, H, Chen, L, Wang, J, (2005), Preliminary study on the inhibition of polysaccharide of edible fungi to plant virus, J Anhui Agric Univ, 32(1), p15-18.
-
Zhang, Y, Ahn, BY, (2015), Optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in shitake mushrooms (Lentinula edodes) by using response surface methodology, J Appl Biol Chem, 58(1), p25-29.
[https://doi.org/10.3839/jabc.2015.006]